This is one of those posts which I am not sure if I am interpreting the results correctly but if I am, I would say that it may lead to an entire area of research that we can go into and really have a good chance at causing epiphyseal growth plate regeneration in a minimal invasive approach.
Recently I came across a PubMed study which has made me question whether the entire growth plate physiology we have been learning may be missing a critical piece. That piece was that the distinct layers of the growth plate where the individual chondrocytes wll move through in its life cycle don’t just move in one direction, but can go also in reverse. The study is entitled “Parathyroid hormone [PTH(1-34)] and parathyroid hormone-related protein [PTHrP(1-34)] promote reversion of hypertrophic chondrocytes to a prehypertrophic proliferating phenotype and prevent terminal differentiation of osteoblast-like cells.”
The abstract is below…
J Bone Miner Res. 1999 Aug;14(8):1281-9.
Parathyroid hormone [PTH(1-34)] and parathyroid hormone-related protein [PTHrP(1-34)] promote reversion of hypertrophic chondrocytes to a prehypertrophic proliferating phenotype and prevent terminal differentiation of osteoblast-like cells.
Source
Istituto Nazionale per la Ricerca sul Cancro, Centro di Biotecnologie Avanzate, Genova, Italy.
Abstract
The effects of parathyroid hormone/parathyroid hormone-related protein (PTH/PTHrP) on late events in chondrocyte differentiation were investigated by a dual in vitro model where conditions of suspension versus adhesion culturing are permissive either for apoptosis or for the further differentiation of hypertrophic chondrocytes to osteoblast- like cells. Chick embryo hypertrophic chondrocytes maintained in suspension synthesized type II and type X collagen and organized their extracellular matrix, forming a tissue highly reminiscent of true cartilage, which eventually mineralized. The formation of mineralized cartilage was associated with the expression of alkaline phosphatase (ALP), arrest of cell growth, and apoptosis, as observed in growth plates in vivo. In this system, PTH/PTHrP was found to repress type X collagen synthesis, ALP expression, and cartilage matrix mineralization. Cell proliferation was resumed, whereas apoptosis was blocked. Hypertrophic chondrocytes cultured in adherent conditions in the presence of retinoic acid underwent further differentiation to osteoblast-like cells (i.e., they resumed cell proliferation, switched to type I collagen synthesis, and produced a mineralizing bone-like matrix). In this system, PTH addition to culture completely inhibited the expression of ALP and matrix mineralization, whereas cell proliferation and expression of type I collagen were not affected. These data indicate that PTH/PTHrP inhibit both the mineralization of a cartilage-like matrix and apoptosis (mimicked in the suspension culture) and the production of a mineralizing bone-like matrix, characterizing further differentiation of hypertrophic chondrocytes to osteoblasts like cells (mimicked in adhesion culture). Treatment of chondrocyte cultures with PTH/PTHrP reverts cultured cells in states of differentiation earlier than hypertrophic chondrocytes (suspension), or earlier than mineralizing osteoblast-like cells (adhesion). However, withdrawal of hormonal stimulation redirects cells toward their distinct, microenvironment-dependent, terminal differentiation and fate.
PMID: 10457260 [PubMed – indexed for MEDLINE]
Analysis: While the study was done on chickens, I personally believe that the same PTH/PTHrP feedback loop is also seen in humans. It says very clearly that the PTH/PTHrP hormonal balance managed to inhibit the formation of compounds only the hypertrophic chondrocytes release, which are the Collagen Type X, the ALP, and mineralization. It says very clearly…”PTH/PTHrP was found to repress type X collagen synthesis, ALP expression, and cartilage matrix mineralization. Cell proliferation was resumed, whereas apoptosis was blocked.”
If we remember from studies we looked at in the past, specifically “BMP and Ihh/PTHrP signaling interact to coordinate chondrocyte proliferation and differentiation.” The two main points were…
- During endochondral ossification, two secreted signals, Indian hedgehog (Ihh) and parathyroid hormone-related protein (PTHrP), have been shown to form a negative feedback loop regulating the onset of hypertrophic differentiation of chondrocytes
- Overexpression of Ihh in the cartilage elements of transgenic mice results in an upregulation of PTHrP expression and a delayed onset of hypertrophic differentiation.
 Now, let’s look at this other study entitled “The PTHrP–Ihh feedback loop in the embryonic growth plate allows PTHrP to control hypertrophy and Ihh to regulate proliferation”
Now, let’s look at this other study entitled “The PTHrP–Ihh feedback loop in the embryonic growth plate allows PTHrP to control hypertrophy and Ihh to regulate proliferation”
The abstract is below…
Growth plate and long bone development is governed by biochemical signaling pathways of which the PTHrP–Ihh system is the best known. Other factors, such as BMPs, FGFs and mechanical loading, may interact with this system. This study aims at elucidating the relative importance of PTHrP and Ihh for controlling proliferation, and hypertrophy in fetal growth plate cartilage. We assessed the question why reduced Ihh expression leads to more pronounced effects on the number of non-hypertrophic cells and total bone formation, compared to PTHrP down-regulation. Using few basic equations, constituted from literature data, this paper shows how the PTHrP–Ihh feedback system can control different aspects of tissue differentiation at distinct locations. In particular, it is shown that (mechanical or biochemical) perturbations will affect proliferation via Ihh-related parameters, whereas changes in PTHrP-related parameters selectively interact with hypertrophy. This is contra-intuitive, since PTHrP acts to keep cells proliferating. In this context, the critical PTHrP level for keeping cells proliferating has been reconsidered. In addition, an explanation is provided for the aforementioned difference in effect between reduced Ihh and PTHrP expression.
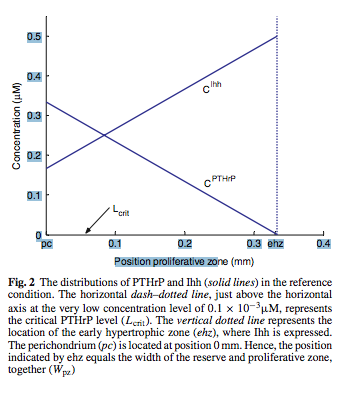 Analysis: From just the abstract and the clipping, it would suggest that the PTHrP is the main component that is known today which determines how much the chondrocytes in the pre-hypertrophic layer and the proliferative layer can replicate. However, after I read further into the dicussion part of the study, it seems that the authors are suggesting that while the PTHrP and the iHH are in a feedback loop where they affect each other, they actually have different roles in different section where it is actually the iHH is what determines the proliferative capacity and the PTHrP is what determines the hypertrophic ability. Of course we have to realize that it would appear the scientists reached this conclusion from mathematical derivation in looking at local, very simplified concentration gradients.
Analysis: From just the abstract and the clipping, it would suggest that the PTHrP is the main component that is known today which determines how much the chondrocytes in the pre-hypertrophic layer and the proliferative layer can replicate. However, after I read further into the dicussion part of the study, it seems that the authors are suggesting that while the PTHrP and the iHH are in a feedback loop where they affect each other, they actually have different roles in different section where it is actually the iHH is what determines the proliferative capacity and the PTHrP is what determines the hypertrophic ability. Of course we have to realize that it would appear the scientists reached this conclusion from mathematical derivation in looking at local, very simplified concentration gradients.
Note to the readers:
- The Ihh is expressed in the pre-hypertrophic chondrocytes
- The PTHrP is expressed in the perichondrium
J Cell Biochem. 2001;80(4):504-11.Parathyroid hormone-related peptide expression in the epiphyseal growth plate of the juvenile chicken: evidence for the origin of the parathyroid hormone-related peptide found in the epiphyseal growth plate.
Source
Department of Poultry Science, 213 William L. Henning Building, The Pennsylvania State University, University Park, PA 16802, USA.
Abstract
Parathyroid hormone-related peptide (PTHrP) has been shown to be essential for normal endochondral bone formation. Along with Indian hedgehog (Ihh), it forms a paracrine regulatory loop that governs the pace of chondrocyte differentiation. However, the source of PTHrP for this regulatory loop is not clear. While one hypothesis has suggested the periarticular perichondrium as the source of PTHrP for growth plate regulation, other data utilizing immunohistochemistry and in situ hybridization would indicate that growth plate chondrocytes themselves are the source of this peptide. The data described in this report supports the view that postnatal growth plate chondrocytes have the ability to synthesize this important regulatory peptide. Immunohistochemistry of tissue sections showed that PTHrP protein was evident throughout the chick epiphysis. PTHrP was seen in chondrocytes in the periarticular perichondrium, the perichondrium adjacent to the growth plate, the prehypertrophic zone of the growth plate, and the hypertrophic zone of the growth plate. However, cells in the proliferative zone, as well as some chondrocytes in the deeper layers of articular cartilage were predominantly negative for PTHrP. PTHrP was detected by Western blotting as a band of 16,400 Da in extracts from hypertrophic chondrocytes, but not from proliferative cells. RT-PCR detected PTHrP mRNA in both proliferative and hypertrophic growth plate chondrocytes, as well as in articular chondrocytes. PTH/PTHrP receptor mRNA was detected by Northern blotting in growth plate, but not articular chondrocytes. Thus, we conclude that most of the PTHrP present in the epiphyseal growth plate of the juvenile chick originates in the growth plate itself. Furthermore, the presence of large amounts of PTHrP protein in the hypertrophic zone supports the concept that PTHrP has other functions in addition to regulating chondrocyte differentiation.
Copyright 2001 Wiley-Liss, Inc.
PMID: 11169734 [PubMed – indexed for MEDLINE]
Personal Interpretation: Let’s assume that the study on chickens is the same as humans, since the other PubMed studies done on lab animals like rats showed a similar negative paracrine feedback loop. This means that we know where the Parathyroid hormone-related peptide is coming from, the growth plates themselves. Sure, they might be coming from somewhere else as well, like the adrenal glands or the thyroid glands, however the majority of the PTHrP that makes up the PTH/PTHrP and iHH loop comes from the growth plates. The PTH obviously comes from the parathyroid glands. The researchers found the PTHrP mRNA in two areas, the hypertrophic area, and the proliferative layer. We saw in the previous study that the researchers believed the PTHrP is coming from the perichondrium, closer to the articular cartilage region. However this study seems to disapprove that view.
The big thing that is very interesting is that you don’t find the PTHrP in the articular cartilage, but only the epiphyseal cartilage.
Let’s now look at the Wikipedia article on the Parathyroid hormone related peptide. “It is occasionally secreted by cancer cells (breast cancer, certain types of lung cancer including squamous cell carcinoma)…It regulates endochondral bone development by maintaining the endochondral growth plate at a constant width….PTHrP is related in function to the “normal” parathyroid hormone. When a tumor secretes PTHrP, this can lead to hypercalcemia.[3] As this is sometimes the first sign of the malignancy.”
From the Wikipedia article on Hypercalcemia, we learn that hypercalcemia is “is an elevated calcium level in the blood.” How this condition is related back to the PTH/PTHrP where the PTH/PTHrP ratio and balance has a main function where it controls how much calcium is supposed to either be in the blood stream or be absorbed to the inorganic bone matrix making it harder.
In my personal research I have actually found a weak inverse correlation between bone density and bone length. Since the calcium absorption to the bone is correlated to bone density, if the PTHrP is causing the calcium to be lost from the bone matrix, that means that the bone is weaker, so it is weaker allowing it to be more easily modified (ie. lengthened). What is given is that its mechanical properties which measure strength of the bone is smaller.
Remember also that we have found a rather strong correlation between great height and increase cancer risk. What I believe is that we have found the very first major compound which can lead to possible epiphyseal plate regrowth en vivo/ endogenously. However a huge warning must be put to this post because of the nature of what we are intending to do. Nature is wise in keeping humans and other animals to a certain size. It has many mechanisms that turn on so that e don’t overgrow out of bounds. Technically, any growth of bone size after cartilage closure probably has an extremely high risk and chance for developing into forms of cancer.
The idea is really just get more PTHrP into the growth plate, since it seems to have a positive correlation to keeping the growth plate thickness intact, and has the ability to reverse the hypertrophic chondrocytes back to the proliferative stage. This means that the growing age range can be extended, as long as the person has some chondrocytes in the resting zone left. The idea is to use two types of injection needles. One is to add new progenitor cells into the resting zone so that there will be more initial raw material (ir. progenitor chondorcytes) to work with. The 2nd injection is the PTHrP, which would cause the chondrocytes to focus more on proliferation and actually keep them from hypertrophizing. The 3rd injection would be for the iHH which would cause the iHH expression to increase and push the process of chondrocye differentiation back. Adding a fourth injection of other growth factors like BMPs and FGF-2 would lead to the chondrocytes to proliferate even more. All that is required after that is for the chondrocytes to reach past the endochondral layer and get to the outside to get a chance to push in the right direction. If we do not keep the growth direction correctly, it could lead to just forms of chondrocsarcoma and osteosarcoma.
This method to regrow the plate over again is the first time I have seen that the width of the plate can be increased again. The fact that it says very clearly in the Wikipedia article that the PTHrP is secreted by cancer cells shows that this is the type of compound that would be needed to restart the cartilages again.

This is quite big!
How has something like this been overseen? Is there really nobody out there doing this research, other than you and Tyler?
The flouride in our water might have an effect on our height, according to this:
http://www.biomedsearch.com/nih/Elevation-PTH-PTHrp-Induced-by/19915804.html
Hmm…I believed that Ihh was more more important to restart growth plates than PTH.
You are right about that issue. At some point, it seems to point to the fact that maybe iHH is the starting initiating compound. However all the studies I have found are pointing at the fact that PTHrP can stop and potentially reverse the chondrocyte life cycle of differentiation. This means we can hold off on ossification, and cause more proliferation. Plus, the link with PTHrP with cancer shows that it can reinitiate cell proliferation (even thought is is uncontrolled) beyond the natural state.
One more thing is that growth plate chondrocytes don’t transdifferentiate into osteoblasts, GP chondrocytes undergo apoptosis and other cell death mechanisms. So even though PTH decreases maturity in the GP, the majority of the GP cells die so dedifferentiating osteoblasts won’t help as they don’t have the GP chondrocytes epigenetic material like they would if they transidfferentiated into osteoblasts.
What you need to do is get new stem cells to differentiate into chondrocytes.
I agree with you. If increase the population of mesenchymal stem cells, towards the chondrocyte lineage, we may effectively increase the width of the epiphyseal plate. I’ve found a couple resources which state that MSC proliferation is regulated by FGFs, TGF beta and PDGF (just a few to name). The link to one article is http://stemcellres.com/content/1/4/32. However, does anyone know how we would go about increasing the production of these hormones in the body?
I’ll look into that.
Pingback: How To Use PTHrP, PPR, and IHH To Increase Height And Grow Taller