This study is huge because we can get adult epithelial cells to potentially form new growth plates. Since the resting zone is composed of mainly endothelial-like cells and the resting zone is the foundation of the growth plate. Understanding how to transition adult mesenchymal stem cells into endothelial cells may be the basis for forming a new growth plate.
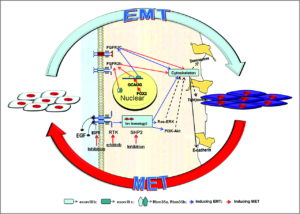
“For linear longitudinal bone elongation, the stem-like progenitor chondrocytes distributed in resting zone (RZ) of growth plate have a capacity to differentiate towards the spindle chondrocytes in proliferative zone (PZ), then towards the columnar and tightly adjacent chondrocytes in hypertrophic zone (HZ). We hypothesized this process of endochondral ossification with cells morphological change was occurred along with the inter-conversion between epithelial to mesenchymal cell types. Consistent with this hypothesis, the chondrocytes highly expressed mesenchymal-like biomarkers and loss of epithelial surface markers in PZ, while converse in RZ and HZ of the growth plate in mice distal tibia in vivo. The 4-week old male and female mice were treated with estradiol cypionate or oxandrolone, then investigated the response of epithelial- and mesenchymal biomarkers, and demonstrated that estrogen blocked the EMT process from RZ to PZ while androgen promoted MET from PZ to HZ. Our observations supported the hypotheses that the growth plate firstly go through EMT from RZ to PZ, then MET process from PZ to HZ during the epiphyseal fusion. Our results could interpret the different roles of estrogen and androgen in growth plate cartilage [undergoing] endochondral ossification.”
In Epiphelial to Mesenchymal Cell Transition, cells lose cell-cell adhesion and cell polarity properties to become more migratory mesenchymal cells. Since the growth plate firstly grows via Epipthelial to Mesenchymal Cell Transitiation at the RZ to PZ that is the most important stage for us to focus on as that causes the formation of the growth plate and the formation of the growth plate would be a great help in growing taller. However, the cell condensation stage to establish the resting cell zone should first require epithelial cell types as cell to cell adhesion would be needed.
“Multiple tissues differentiation and organs formation in embryonic development arise from a
series of conversion from epithelial to mesenchymal cells, through epithelial to mesenchymal
transition (EMT) or mesenchymal to epithelial transition (MET). In primary EMT process,
the primitive epithelia lose their characterization of rounded shape, sequential arrangement and compact junctions to convert a population of spindle, loosely organized but motile mesenchymal cells{hydrostatic pressure may help organize the stem cells and change the structure of these cells} for gastrulation formation and neural crest migration. Then, after a transient epithelial structure condensation through MET, these population in notochord, somites, somatopleure and splanchnopleure derived from mesoderm generate mesenchymal cells which have ability to differentiate into specific cells types of diverse tissues via the secondary EMT”
” the neural crest cells migrate to somites of mesoderm following stereotyped pathways and undergo a secondary EMT to generate mesenchymal condensation. These mesenchymal cells differentiate into osteoprogenitors for intramembranous ossification and chondrocytes for endochondral ossification. ”
” Pluripotent stem cells exhibit epithelial characteristics, down-regulate the epithelial markers such as Cdh1, Cldn6, Epcam and enhance the mesenchymal markers including Snai1/2, Zeb1,
CtnnbIP1″
” estrogen administration maintains the epithelial type genes expression in growth plate particularly in RZ implies that estrogen appears to block EMT process.”
“Not like human or rabbit, the expression of estrogen receptors within HZ of growth plate in mice and rat was extremely low until at the last time point prior to epiphyseal fusion, which also
reflects the less effect of estradiol cypionate to in the HZ in our study. Conversely, Androgen
effectively promotes EMT for chondrocytes differentiation”
” estrogen may interdict TGF-beta, then further repress Smad3 expression, so that
postpone chondrocytes differentiation via EMT blocking. ”
“Androgen is determined to promote EMT for differentiation. However, androgen improves Smad3 expression but appears to have no response to SIS3, which indicates that androgen may participate in other pathways rather than TGF-beta/Smad3.”
” A notable presence of growth plate was observed in distal tibia of the 4-week but not in 16-week old mice”<-Note that LSJL has worked in 16-week old mice. Although in the LSJL study they used Sprauge -Dawley rats in contrast to outbred ICR mice.
” The mRNA level of epithelial markers including Cdh1, Cldn6, Col4a1, Krt19, Lamc1 expressed in RZ and HZ were significantly higher than in PZ while the mesenchymal markers such as Acta2, Ctnnb1, Smad3, displayed the converse tendency. The results suggested that a process of EMT occurred in the programming of RZ towards PZ and MET in PZ towards HZ.”
Here’s the 16-week old growth plate, still present but weak:
Epithelial cells may already exist in adult bone marrow.
Epithelial cells in bone marrow: do they matter?
“epithelial-like cells can be detected in the bone marrow of many patients not known to have cancer. ”
Inducing a mesenchymal to epithelial transition would be difficult as I haven’t found any studies of it occuring due to physiological stimuli. Another possibility though would be to have a growth plate without a resting zone as the proliferative zone consists mainly of mesenchymal cells. The viability of this depends on the viability of a growth plate without a resting zone. The resting zone may play a role in growth plate orientation which makes sense as epithelial cells tend to be involved in cell polarity and cell adhesion.
Here’s a diagram of the mesenchymal-epithelial transition:
LSJL upregulates Pcdhb2(protocadherin beta 2), Cdh13, Ctnna3, Fat1(a cell adhesion model). It downs regulates protocadherin subfamily A, 4(Pcdhga4), AK002616(a miscellaneous Cadherin related protein), Celsr2, Cdh15, Cdh11. So LSJL has the definite potential to affect the mesenchymal-epithelial transition although how isn’t clear as it affects a lot of related genes but not in a clear pattern
For the other parts LSJL downregulates Cldn13, Dsp(Desmoplakin isoform 1, the downregulation of this gene suggests that LSJL likely encourages the epithelial to mesenchymal transition but that doesn’t mean that it doesn’t encourage the mesenchymal to epithelial transition as well), and upregulates a gene related to Cldn19, Muc3. It also downregulates an anti-mucin gene.
This diagram mentions the reverse markers:
According to Actin stress fibres and cell-cell adhesion molecules in tendons: organisation in vivo and response to mechanical loading of tendon cells in vitro.
“Tendons consist of parallel longitudinal rows of cells separated by collagen fibres. The cells are in intimate contact longitudinally within rows, and laterally via sheet-like lateral cell processes between rows. At points of contact, they are linked by gap junctions. Since tendons stretch under load, such cell contacts require protection. Here we describe the organisation of the actin cytoskeleton and actin-based cell-cell interactions in vivo and examine the effect of cyclic tensile loading on tendon cells in vitro. Cells within longitudinal rows contained short longitudinally running actin stress fibres. Each fibre was aligned with similar fibres in the cells longitudinally on either side, and fibres appeared to be linked via adherens junctions. Overall, these formed long oriented rows of stress fibres running along the rows of tendon cells. In culture, junctional components n-cadherin{this increase is actually not good news for creation of epithelial cells as this is a mesenchymal marker} and vinculin and the stress fibre component tropomyosin increased in strained cultures, whereas actin levels remained constant. (1) cells are linked via actin-associated adherens junctions along the line of principal strain; and (2) under load, cells appear to attach themselves more strongly together, and assemble more of their cytoplasmic actin into stress fibres with tropomyosin. Cell-cell contacts are protected during stretch, and also that the stress fibres, which are contractile, may provide an active mechanism for recovery from stretch. In addition, stress fibres are ideally oriented to monitor tensile load and thus may be important in mechanotransduction and the generation of signals passed via the gap junction network.”
So according to this in response to load cells may establish more cell-cell contact characteristic of epithelial cells.
“epithelial characteristics were dramatically associated with increased bone and soft-tissue colonization after intracardiac or intratibial injection.”
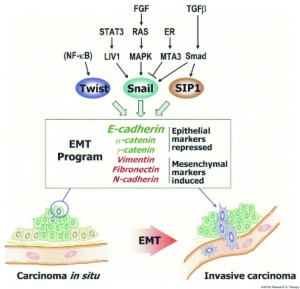
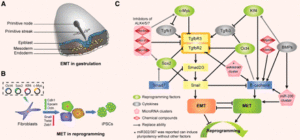
“Multiple complex signaling systems are required for the induction of EMT and are also closely related with MET. The FGFR2 gene, which is located at human chromosome 10q26, encodes for FGFR2b and FGFR2c isoforms due to alternative splicing and mutually exclusive use of exon IIIb or exon IIIc. FGFR2b primarily binds FGF10 and FGF7 and is the isoform of choice in epithelial cells, whereas FGFR2c binds FGF2 and is mainly expressed in cells of mesenchymal origin. FGF/FGFR2 signaling governs the EMT that is required for organogenesis in mouse embryos.”
“expression of FGFR2b induced MET [induced cancer in one instance]”
“As for the regulation of FGFR2 isoforms’ alternative splicing, a highly conserved GCAUG element was shown to be required for efficient exon IIIb activation. Afterward, Fox protein family members, especially Fox-2, were shown to regulate the FGFR2 exon choice, and this regulation was absolutely dependent on the GCAUG elements present in the FGFR2 pre-mRNA. Fox-2 induced the FGFR2c to FGFR2b switch, accompanied by molecular and morphological changes consistent with MET”
“2 paralogous epithelial cell type–specific RNA binding proteins, Rbm35a and Rbm35b, which are essential regulators of FGFR2 splicing. Ectopic expression of either protein in cells that express FGFR2c caused a switch in endogenous FGFR2 splicing to the epithelial isoform”<-Note it’s FGFR3 that’s typically associated with dwarfism.
EMT and MET as paradigms for cell fate switching
“Cell fate determination is a major unsolved problem in cell and developmental biology. The discovery of reprogramming by pluripotent factors offers a rational system to investigate the molecular mechanisms associated with cell fate decisions. The idea that reprogramming of fibroblasts starts with a mesenchymal-epithelial transition (MET) suggests that the process is perhaps a reversal of epithelial to mesenchymal transition (EMT) found frequently during early embryogenesis. As such, we believe that investigations into MET-EMT may yield detailed molecular insights into cell fate decisions, not only for the switching between epithelial and mesenchymal cells, but also other cell types.”
“In any given animal tissue, one may find two very common cell types: the epithelial cells that line the surface of a tissue or organ and mesenchymal cells that are embedded in the three-dimensional matrix. “<-Growth plate cells line the bone matrix.
“the epithelial cells are attached to the basement membrane, establish an apical–basal axis of polarity, and communicate with each other through the gap junction. Across and underneath the basement membrane, there is the stroma made of the three-dimensional extracellular matrix synthesized by the resident mesenchymal or stromal cells.”
“Inside the nuclei, Snail genes are considered as the most important downstream targets of the nodal-SMAD2/3 pathway during gastrulation. The Snails may in fact be the guardians of the mesenchymal phenotype by activating mesenchymal genes and suppressing epithelial genes. Indeed, Snails have been shown to down-regulate E-cadherin effectively, which is one of hallmarks for epithelial cells. Snail-deficient embryos could not proceed through gastrulation and form mesodermal cells as they could not down-regulate the expression of E-cadherin in the primitive streak. It is generally recognized that the embryonic EMT process is orchestrated and maintained through the collaboration of extracellular signals and intracellular transcription factors.”

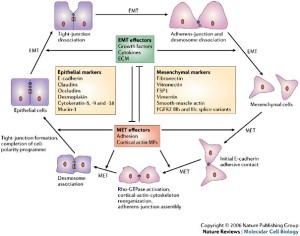
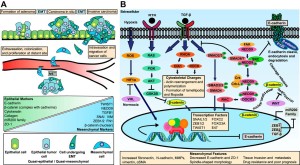
I have a question about epipheseal plate.
When the epiphyseal plate closes, it forms a epiphyseal line. Is epiphyseal line a compact bone tissue?
it’s hard to have patience…
they can grow working vocal cord tissue but can’t grow growth plates?
http://news.sciencemag.org/biology/2015/11/scientists-grow-working-vocal-cord-tissue-lab
Finally had time to read through this article. ..
Your theory sounds like you want to induce a metastatic cancer lol. Even if we have EMT successfully transformed (physically, chemically), how do we get those cells to settle within the epiphysis without getting crushed?
One more thought. Do you mind doing a post about how growth plate closes in detail? I think it’s essential that we know how closure occurs if we want to come up with a method to reverse the process.
I have a strong feeling that it has something to do with telomeres. Like when chondrocytes stops to differentiate due to the shortage of telomeres, that’s when growth plate closes and become a epiphyseal line.
I think if when the growth plates close down, it leaves an epiphyseal line which I think personally is due to hormones and can be reversed through hypnosis. I am gonna research in details about all this phenomena and try to crack the code.
Hormones play a part but it is usually a very specific mutation required to increase longitudinal bone growth.
With hypnosis, you’d have to prove that it actually alters hormones or the microenvironment of the bone.
The thing about telomeres is that there haven’t been any telomere related genes that have been found to be related to increased longitudinal bone growth. The main development genes associated with increased longitudinal bone growth are HMGA and IGF2. I think there are other limiting factors that come into play before telomeres. So if you extend longitudinal bone growth via other methods then telomeres become a factor.
Also, chondrocyte hypertrophy generally shows stronger effects on height than chondrocyte proliferation.
Tyler, you are doing a great job but I have a question why don’t you look into the spine? I mean it seems the most easy place to alter the height you should rather look into spine and find out if we can get atleast 3 inches then the problem of height will be solved easily.