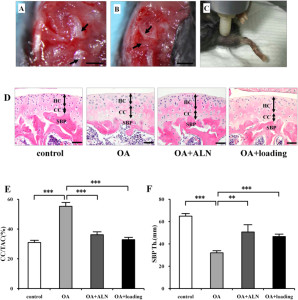
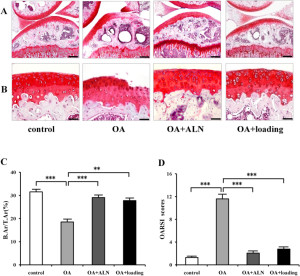
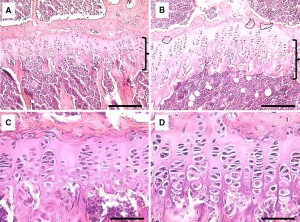
This study is focused mainly on bone formation but it looks at MSCs in general and stimulating stem cells would be an important step in growing taller.
Dynamic Fluid Flow Mechanical Stimulation Modulates Bone Marrow Mesenchymal Stem Cells.
“Osteoblasts are derived from mesenchymal stem cells (MSCs){Also chondrocytes are derived from MSCs}, which initiate and regulate bone formation. New strategies for osteoporosis treatments have aimed to control the fate of MSCs. While functional disuse decreases MSC growth and osteogenic potentials, mechanical signals enhance MSC quantity and bias their differentiation toward osteoblastogenesis. Through a non-invasive dynamic hydraulic stimulation (DHS), we have found that DHS can mitigate trabecular bone loss in a functional disuse model via rat hindlimb suspension (HLS). To further elucidate the downstream cellular effect of DHS and its potential mechanism underlying the bone quality enhancement, a longitudinal in vivo study was designed to evaluate the MSC populations in response to DHS over 3, 7, 14, and 21 days. Five-month old female Sprague Dawley rats were divided into three groups for each time point: age-matched control, HLS, and HLS+DHS. DHS was delivered to the right mid-tibiae with a daily “10 min on-5 min off-10 min on” loading regime for five days/week. At each sacrifice time point, bone marrow MSCs of the stimulated and control tibiae were isolated through specific cell surface markers and quantified by flow cytometry analysis. A strong time-dependent manner of bone marrow MSC induction was observed in response to DHS, which peaked on day 14. After 21 days, this effect of DHS was diminished. the MSC pool is positively influenced by the mechanical signals driven by DHS. Coinciding with our previous findings of mitigation of disuse bone loss, DHS induced changes in MSC number may bias the differentiation of the MSC population towards osteoblastogenesis, thereby promoting bone formation under disuse conditions. the mechanism of MSC induction in response to mechanical loading [is time sensitive], and for the optimal design of osteoporosis treatments. ”
Whether MSCs are driven towards osteo or chondrogenesis could be related to the microenvironment. So how strong the existing bone is could be a key as to whether the MSCs are driven torwards chondro or osteogenesis.
“DHS was delivered through a custom-made inflatable cuff placed around the right hind limb above the tibia.{so maybe occlusion bands could help?} An oscillatory actuator-driven syringe, a force-controlled syringe and a pressure sensor, were connected to the stimulation cuff. The actuator-driven syringe was controlled by a programmable 100 MHz waveform/signal generator. The hydraulic pressure was monitored by the pressure sensor throughout the entire treatment. With a stimulation frequency of 2 Hz, the pressure stimulation magnitudes were 30 mmHg static pressure + 30 mmHg (peak-to-peak) dynamic pressure. Daily stimulation of the “10 min on-5 min off-10 min on” loading regime was applied to each stimulated animal, while under anesthesia (isoflurane inhalation), for five days/week.”<-This is less than 0.01MPa well below the 0.1 to 10MPa to induce chondrogenesis.
Hydraulic Stimulation increased the number of bone marrow by 50%.
Dynamic fluid flow induced mechanobiological modulation of in situ osteocyte calcium oscillations.
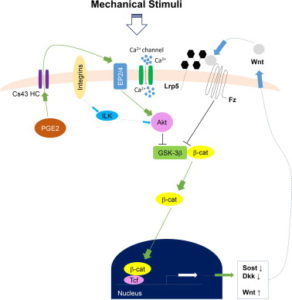
“Distribution of intramedullary pressure (ImP) induced bone fluid flow has been suggested to influence the magnitude of mechanotransductory signals within bone. As osteocytes have been suggested as major mechanosensors in bone network, it is still unclear how osteocytes embedded within a mineralized bone matrix respond to the external mechanical stimuli derived from direct coupling of dynamic fluid flow stimulation (DFFS). While in vitro osteocytes show unique Ca(2+) oscillations to fluid shear, the objective of this study was to use a confocal imaging technique to visualize and quantify Ca(2+) responses in osteocytes in situ under DFFS into the marrow cavity of an intact ex vivo mouse femur. This study provided significant technical development for evaluating mechanotransduction mechanism in bone cell response by separation of mechanical strain and fluid flow factors using ImP stimulation, giving the ability for true real-time imaging and monitoring of bone cell activities during the stimulation. Loading frequency dependent Ca(2+) oscillations in osteocytes indicated the optimized loading at 10Hz, where such induced response was significantly diminished via blockage of the Wnt/β-catenin signaling pathway. The results provided a pilot finding of the potential crosstalk or interaction between Wnt/β-catenin signaling and Ca(2+) influx signaling of in situ osteocytes in response to mechanical signals. Findings from the present study make a valuable tool to investigate how in situ osteocytes respond and transduce mechanical signals, e.g. DFFS, as a central mechanosensor.”<-Our main concern isn’t Ca2+ oscillations as those are not likely to induce chondrogenesis directly although it’s still possible that it may have an impact or that Ca2+ could be complementary.
“Changes in the pressure or velocity of bone fluid flow (BFF) act as a communication medium that connects external loading signals and internal cellular activities in bone, which ultimately regulate the bone remodeling process”<-can we manipulate this loading signals and cellular activities in such a way that we grow taller?
“DHS[Dynamic Hydraulic Stimulation] generated local ImP that acted independently from simultaneous bone strain.”
Here’s a diagram of how Calcium signaling may alter a cell. So Ca2+ is anabolic but it doesn’t really seem to have a direct impact on what lineage a cell traverses(osteo- versus chondro-).
“Interstitial bone fluid flow (IBFF) is suggested as a communication medium that bridges external physical signals and internal cellular activities in the bone, which thus regulates bone remodeling. Intramedullary pressure (ImP) is one main regulatory factor of IBFF and bone adaptation related mechanotransduction. Our group has recently observed that dynamic hydraulic stimulation (DHS), as an external oscillatory muscle coupling, was able to induce local ImP with minimal bone strain as well as to mitigate disuse bone loss. The current study aimed to evaluate the dose dependent relationship between DHS’s amplitude, i.e., 15 and 30mmHg, and in vivo ImP induction, as well as this correlation on bone’s phenotypic change. Simultaneous measurements of ImP and DHS cuff pressures were obtained from rats under DHS with various magnitudes and a constant frequency of 2Hz. ImP inductions and cuff pressures upon DHS loading showed a positively proportional response over the amplitude sweep. The relationship between ImP and DHS cuff pressure was evaluated and shown to be proportional, in which ImP was raised with increases of DHS cuff pressure amplitudes. A 4-week in vivo experiment using a rat hindlimb suspension model demonstrated that the mitigation effect of DHS on disuse trabecular bone was highly dose dependent and related to DHS’s amplitude, where a higher ImP led to a higher bone volume. This study suggested that sufficient physiological DHS is needed to generate ImP. Oscillatory DHS, potentially induces local fluid flow, has shown dose dependence in attenuation of disuse osteopenia. ”
“Direct ImP measurements in rats under DHS over a frequency spectrum indicated that its optimal loading at 2Hz can generate maximum ImP of 14.48±3.10mmHg. On the other hand, dynamic components with large loading amplitudes typically result in pronounced osteogenic responses”
“a micro-cardiovascular pressure transducer was carefully inserted into the tibial marrow cavity through a 1mm drilled hole, and was then tightly sealed within the drilled hole”
” the observed ImP inductions were found to be positively proportional to DHS cuff pressure, namely, which indicates promising potential of DHS loading dose dependency on ImP related bone adaptation.”
“DHS loading at 2Hz between 28.41±10.50 mmHg and 33.75±10.69 mmHg cuff pressures can induce significant ImP increases. Coincided with these observations, our 4-week in vivo study showed that DHS loading at 2Hz with 15 mmHg and 30 mmHg dynamic pressure was able to mitigate disuse trabecular bone loss in a rat functional disuse model.”
“At baseline, normal heart beats generated approximately 1mmHg tibial ImP within the marrow cavity.”
Dynamic hydraulic fluid stimulation regulated intramedullary pressure.
“Physical signals within the bone, i.e. generated from mechanical loading, have the potential to initiate skeletal adaptation. Strong evidence has pointed to bone fluid flow (BFF) as a media between an external load and the bone cells, in which altered velocity and pressure can ultimately initiate the mechanotransduction and the remodeling process within the bone. Load-induced BFF can be altered by factors such as intramedullary pressure (ImP) and/or bone matrix strain, mediating bone adaptation. Previous studies have shown that BFF induced by ImP alone, with minimum bone strain, can initiate bone remodeling. However, identifying induced ImP dynamics and bone strain factor in vivo using a non-invasive method still remains challenging. To apply ImP as a means for alteration of BFF, it was hypothesized that non-invasive dynamic hydraulic stimulation (DHS) can induce local ImP with minimal bone strain to potentially elicit osteogenic adaptive responses via bone-muscle coupling. The goal of this study was to evaluate the immediate effects on local and distant ImP and strain in response to a range of loading frequencies using DHS. Simultaneous femoral and tibial ImP and bone strain values were measured in three 15-month-old female Sprague Dawley rats during DHS loading on the tibia with frequencies of 1Hz to 10Hz. DHS showed noticeable effects on ImP induction in the stimulated tibia in a nonlinear fashion in response to DHS over the range of loading frequencies, where they peaked at 2Hz. DHS at various loading frequencies generated minimal bone strain in the tibiae. Maximal bone strain measured at all loading frequencies was less than 8με. No detectable induction of ImP or bone strain was observed in the femur. This study suggested that oscillatory DHS may regulate the local fluid dynamics with minimal mechanical strain in the bone, which serves critically in bone adaptation. These results clearly implied DHS’s potential as an effective, non-invasive intervention for osteopenia and osteoporosis treatments.”
” bone fluid flow (BFF) with altered velocity or pressure acts as a communication media between an external load and the bone cells, which then regulate bone remodeling. In converse, discontinuous BFF can initiate bone turnover and result in osteopenia”<-Initiating bone turnover could be a good thing if bone structure inhibits anabolism so lowering the frequency may be key.
“DHS was achieved through a costume-made inflatable cuff placed around the right tibia”
” the inflation and deflation of the cuff was driven by a syringe pump with the loading magnitudes and frequencies delivered by a programmable waveform/signal generator”
” 1mmHg tibial ImP and 5mmHg femoral ImP were generated by normal heart beat within the marrow cavities.”
” muscle contraction compresses the blood vessels in muscle, which generates an arteriovenous pressure gradient that further increases the hydraulic pressure in skeletal nutrient vessels and amplifies the capillary filtration in bone”
” using oscillatory muscle stimulation (MS), in which oscillatory MS induced maximal ImP at 20Hz. Oscillatory MS was achieved via two disposable needle-sized electrodes inserted into the quadriceps of the stimulated rats. The electrodes were then connected to the waveform generator with 2V supplies to induce muscle contraction”
“Due to the different physical orientations of how oscillatory MS and DHS contact the loaded tissue, as well as the different material densities and viscosities within hard and soft tissues, maximal DHS-induced ImP may result at relatively lower frequency compared to MS. Direct hydraulic coupling may influence bone adaptation in a more physiological frequency range, where normal heart rate is 360 times per minute.”
“Changes of MSC number in response to DHS bias their differentiation towards osteoblastogenesis, leading to bone formation even under disuse conditions”
“increased bone fluid pressure and bone fluid flow regulation are strongly correlated, our current results of DHS-driven ImP induction suggest a possible mechanism that the induced ImP may subsequently enhance bone fluid flow.”