I went through the papers to see if there’s any potential. LPP is linked to HMGA2 which does have height increase potential applications.
<-the title right off the bat suggests potential as anything that suggests cartilage regeneration may be able to increase height if even only in the joints or spinal height.
“Link protein N-terminal peptide (LPP) in extracellular matrix (ECM) of cartilage could induce synthesis of proteoglycans and collagen type II in cartilaginous cells{if the extracellular matrix of the joints or intervertebral discs is thicker that would overall make you taller!}. Cartilage stem/progenitor cells (CSPCs), the endogenous stem cells in cartilage, are important in cartilage degeneration and regeneration. We hypothesized that LPP could be a stimulator for stem cell-based cartilage regeneration by affecting biological behaviors of CSPC. CSPCs were isolated from rat knee cartilage. We evaluated the promoting effect of LPP on proliferation, migration, and chondrogenic differentiation of CSPCs. The chondrogenic differentiation-related genes and proteins were quantitated. Three-dimensional culture of CSPC was conducted in the presence of TGF-β3 or LPP, and the harvested pellets were analyzed to assess the function of LPP on cartilage regeneration. LPP stimulated the proliferation of CSPC and accelerated the site-directional migration. Higher expression of SOX9, collagen II, and aggrecan were demonstrated in CSPCs treated with LPP. The pellets treated with LPP showed more distinct characteristics of chondroid differentiation than those with TGF-β3. LPP showed application prospect in cartilage regeneration medicine by stimulating proliferation, migration, and chondrogenic differentiation of cartilage stem/progenitor cells.”
So there is a possibility of LPP injects in cartilage regions to make people slightly taller of course with caveats as not everything that has potential works. In the paper there’s a lot about degeneration of articular cartilage so they so the potential for application in that area which would in turn result in potential height increase.
“Link protein, a glycoprotein that exists in human intervertebral discs as well as in the articular cartilage, plays an important role in strengthening the binding between aggrecan and hyaluronan. Link protein N-terminal peptide (LPP) is the cleaved N-terminal 16 amino peptide (DHLSDNYTLDHDRAIH) of link protein. LPP was thought to be the functional fragment of link protein as the cross-linker”
If you look at the doses figure 5 there seems to be an equilibrium effect with around 50ng/mL having the equilibrium effect.

This suggests that if you are not deficient in LPP it may have no impact on height whatsoever(LPP already exists in cartilage regions).
So LPP may have no impact on articular cartilage regions(but that doesn’t mean that it wouldn’t) but there is still the possibility of using LPP to induce stem cell differentiation into chondrocytes. But I don’t think the differentiation of stem cells into chondrocytes is the problem. In distraction osteogenesis there is already chondrogenic differentiation. The problem is likely a lack of stem cells in general in the articular and that bone is not capable of interstitial growth. Perhaps LPP could be used as part of microfracture surgery whose goal is to create microfractures to get stem cells to the articular cartilage but the problem is that the cartilage formed is fibrocartilage. So perhaps LPP could be used to make the cartilage purer.
Bone usually heals by bone remodeling perhaps LPP injections could encourage it to heal via endochondral ossification resulting in taller height over time?
“The goal of treating articular cartilage (AC) injury is to regenerate cartilage tissue and to integrate the neo-cartilage with surrounding host cartilage. However, most current studies tend to focus on engineering cartilage; interface integration has been somewhat neglected. An endogenous regenerative strategy that simultaneously increases the recruitment of bone marrow mesenchymal stem cells (BMSCs) and chondrocytes may improve interface integration and cartilage regeneration. In this study, a novel functionalized self-assembling peptide hydrogel (KLD-12/KLD-12-LPP, KLPP) containing link protein N-peptide (LPP) was designed to optimize cartilage repair. KLPP hydrogel was characterized using transmission electron microscopy (TEM) and rheometry. KLPP hydrogel shared a similar microstructure to KLD-12 hydrogel which possesses a nanostructure with a fiber diameter of 25–35 nm. In vitro experiments showed that KLPP hydrogel had little cytotoxicity, and significantly induced chondrocyte migration and increased BMSC migration compared to KLD-12 hydrogel. In vivo results showed that defects treated with KLPP hydrogel had higher overall International Cartilage Repair Society (ICRS) scores, Safranin-O staining scores and cumulative histology scores than untreated defects or defects treated with KLD-12 hydrogel, although defects treated with KLD-12 and KLPP hydrogels received similar type II collagen immunostaining scores. All these findings indicated that the simple injectable functionalized self-assembling peptide hydrogel KLPP facilitated simultaneous recruitment of endogenous chondrocytes and BMSCs to promote interface integration and improve cartilage regeneration, holding great potential as a one-step surgery strategy for endogenous cartilage repair.”
“LPP can induce directional migration of nucleus pulposus cells (NPCs) and cartilage-derived stem cells (CSCs)”
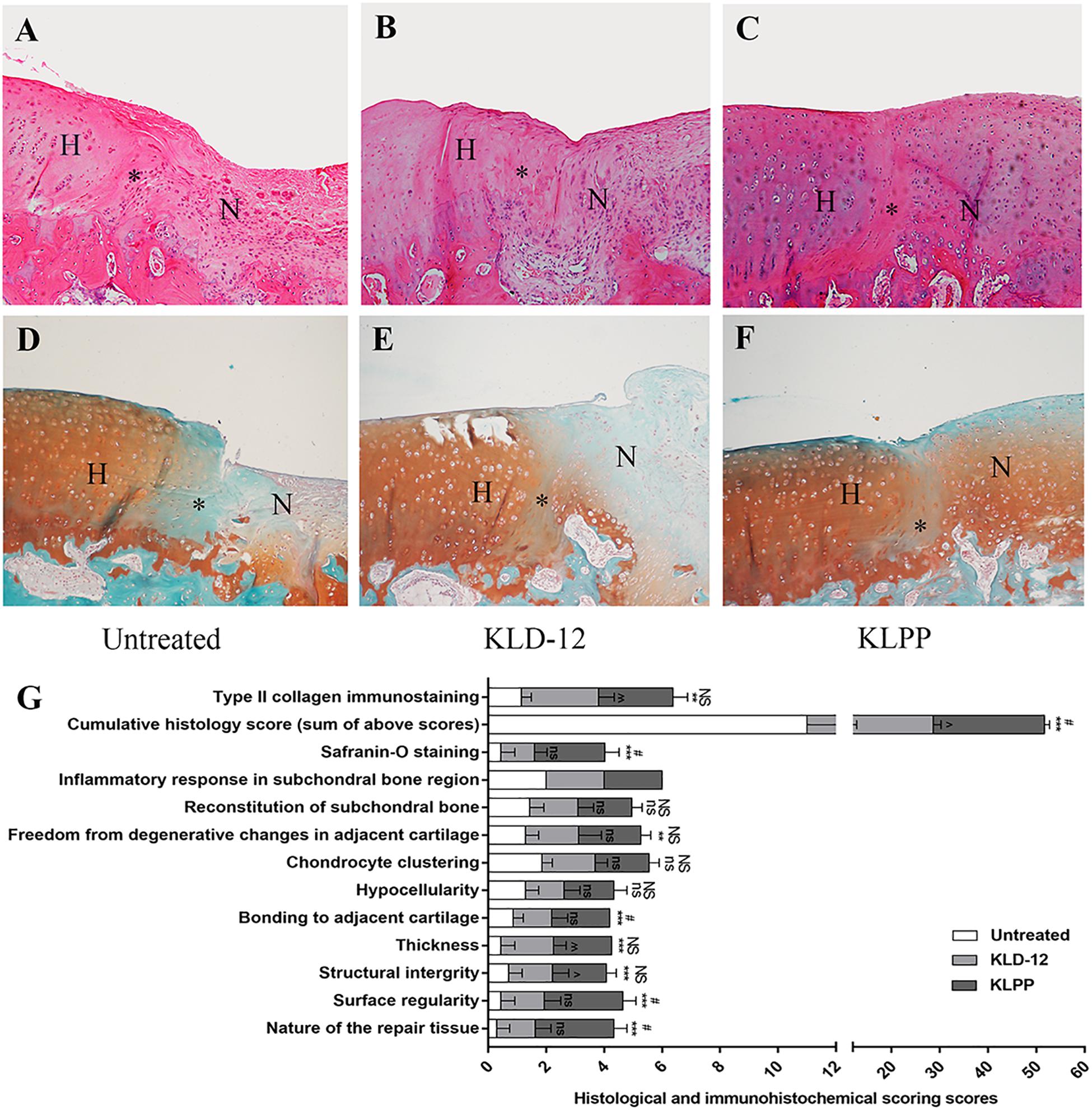
Now is this cartilage “taller” than before the injection? I can’t really tell from the images. IF you look at slide F it looks there might be some slight overgrowth.
Overall I’d say use LPP does have some height increase potential but probably very minor and it’s probably going to be a while before it can be used for height in practice.