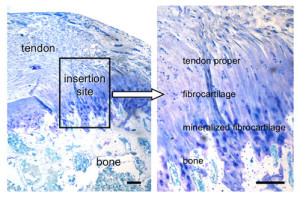
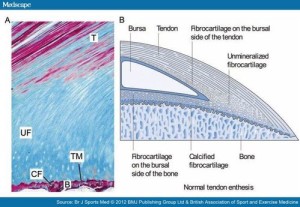
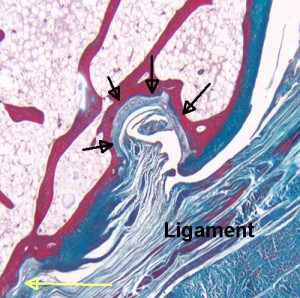
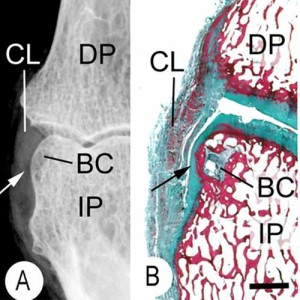
If tensile strain can induce endochondral ossification of articular cartilage then it could help you grow taller.
Micromechanical response of articular cartilage to tensile load measured using nonlinear microscopy.
Articular cartilage of 2-5 year old horses were used. The deep zone was found to be more resistant to tensile strain so it could explain why it’s harder to induce endochondral ossification.
Effects of cyclic tensile strain on chondrocyte metabolism: a systematic review.
“Chondrocytes reorganize the extracellular matrix of articular cartilage in response to externally applied loads. Thereby, different loading characteristics lead to different biological responses. Despite of active research in this area, it is still unclear which parts of the extracellular matrix adapt in what ways, and how specific loading characteristics affect matrix changes. This review focuses on the influence of cyclic tensile strain on chondrocyte metabolism in vitro. It also aimed to identify anabolic or catabolic chondrocyte responses to different loading protocols. The key findings show that loading cells up to 3% strain, 0.17 Hz, and 2 h, resulted in weak or no biological responses. Loading between 3-10% strain, 0.17-0.5 Hz, and 2-12 h led to anabolic responses; and above 10% strain, 0.5 Hz, and 12 h catabolic events predominated{catabolic responses do not necessarily mean a bad thing it could indicate remodeling events that are needed for anabolic responses such as endochondral ossification}. However, this review also discusses that various other factors are involved in the remodeling of the extracellular matrix in response to loading, and that parameters like an inflammatory environment might influence the biological response.”
“after loading with CTS, cells exhibited a more elongated cell shape and aligned perpendicular to the loading direction”<-a more growth plate like organization perhaps?
“Fibronectin connects collagen fibers and other ECM proteins. It is linked to the cell membrane through integrins and might transmit forces from the ECM to the chondrocyte. CTS at 7%, 0.33 Hz and 0.5 Hz, for 4, 12 and 24 h increased the fibronectin mRNA levels in comparison to non-loaded chondrocytes”
“cartilage oligomeric matrix protein (COMP) was increased in response to cyclic tension in chondrocytes”
“a peak hydrostatic pressure of 3.45 MPa occurs in the femoral cartilage during a squat”
“during cartilage compression, the cell´s height (in split line direction) is reduced whereas the cell´s width (perpendicular to the split line) is increased. From the following considerations, one can assume that under physiological cartilage loading this increase in width represents a cell elongation of about 5%”
“in direction of the strain, in uniaxial experiments 79% ± 34% of the strain were transferred to fibroblasts and 63% ± 11% were transferred to tenocytes. In other experiments, 37% ± 8% and 45–60% of biaxial strains were transferred to tenocytes and bone marrow-derived stromal cells”
“in a non-inflammatory environment loading protocols up to 3% cell strain, 0.17 Hz and 2 h could be determined as “low CTS”, between 3–10% cell strain, 0.17 Hz—0.5 Hz and 2–12 h as “moderate CTS” and above 10% cell strain, 0.5 Hz and 12 h as “high CTS”. Loading duration might be the key parameter in triggering gene expression in response to CTS”
“there were no obvious differences between the response of chondrocytes e. g. from the temporomandibular joint and from the knee joints”
Cyclic equibiaxial tensile strain induces both anabolic and catabolic responses in articular chondrocytes.
“Mechanical disturbance is directly implicated in the development of osteoarthritis (OA) but the precise mode for degenerative changes is still largely unknown because of the complexity of the biomechanical and biochemical milieu in the articular joint. To investigate the effects of tensile strain on articular cartilage, cyclic equibiaxial tensile strain (CTS, 0.5 Hz, 10% strain) was applied to monolayer cultures of porcine articular chondrocytes by using a Flexercell strain unit. Overproduction of proinflammatory mediators and imbalanced expression of anabolic and catabolic genes were induced. The cellular secretion of nitric oxide (NO) and prostaglandin E(2) (PGE(2)), as well as the mRNA level of cyclooxygenase-2 (COX-2) were up-regulated in response to mechanical stimuli. Additionally, CTS resulted in an initial peak of anabolic response at 3 h of stretch with respect to the expression of type II collagen and aggrecan. After 12 h of CTS, the expression for these two cartilage-specific matrix proteins fell to control levels. A distinct catabolic response developed after 24 h of stretch with an increase in matrix metalloproteinase-1 (MMP-1). Interestingly, a parallel increase in transforming growth factor (TGF) beta3 was associated with the anabolic changes while an increase in expression of TGF beta1, the predominant isoform of the TGF family, appeared at 24 h. The expression at 24 h of MMP-1, an enzyme that degrades interstitial collagens as well as other cartilage matrix proteins and TGF beta1, may signify a shift towards matrix remodeling and potentially a change in matrix composition as a consequence of continuous CTS.”
The cells did change alignment in response to strain. 6 hours seemed to be the most anabolic stretching time period for cartilage growth but the catabolic genes may be needed for endochondral ossification remodeling. Since only superficial zone was used it’s difficult to tell the effects of inducing endochondral ossification.
Modelling cartilage mechanobiology.
“The growth, maintenance and ossification of cartilage are fundamental to skeletal development and are regulated throughout life by the mechanical cues that are imposed by physical activities. Finite element computer analyses have been used to study the role of local tissue mechanics on endochondral ossification patterns, skeletal morphology and articular cartilage thickness distributions. Using single-phase continuum material representations of cartilage, the results have indicated that local intermittent hydrostatic pressure promotes cartilage maintenance. Cyclic tensile strains (or shear), however, promote cartilage growth and ossification. Because single-phase material models cannot capture fluid exudation in articular cartilage, poroelastic (or biphasic) solid/fluid models are often implemented to study joint mechanics. In the middle and deep layers of articular cartilage where poroelastic analyses predict little fluid exudation, the cartilage phenotype is maintained by cyclic fluid pressure{tensile strain may disrupt this fluid pressure thus disrupting the cartilage phenotype} (consistent with the single-phase theory). In superficial articular layers the chondrocytes are exposed to tangential tensile strain in addition to the high fluid pressure. Furthermore, there is fluid exudation and matrix consolidation, leading to cell ‘flattening’. As a result, the superficial layer assumes an altered, more fibrous phenotype. These computer model predictions of cartilage mechanobiology are consistent with results of in vitro cell and tissue and molecular biology experiments.”
“In a developing cartilage rudiment, one can recognize the same endochondral growth and ossification processes both at the primary ossification front and around secondary ossification sites. There are regions of quiescence that are characterized by the presence of resting chondrocytes. As growth proceeds, these cells proliferate and then mature as they begin to increase the production of ECM, which is characterized by important cartilage molecules, aggrecan and collagen II. As these mature chondrocytes begin to hypertrophy, they express Ihh an important growth factor that upregulates the expression of bone morphogenetic proteins. In the late stages of chondrocyte hypertrophy, the cartilage ECM septa between the columns of hypertrophic chondrocytes calcify. There is increased expression of MMPs (MMP9, MMP13), vascular endothelial growth factor, collagen X and CTGF, in preparation for angiogenesis and ossification. The hypertrophic chondrocytes then undergo apoptosis, and vascular invasion is initiated through their vacant lacuna. Chondroclasts are recruited to the site and begin to resorb the calcified cartilage, eventually destroying two-thirds of the calcified matrix. Perivascular mesenchymal cells differentiate into osteoblasts and begin to form new osteoid on the remaining calcified septa. The osteoid mineralizes to form primary bone trabeculae and the growth and ossification process is complete. ”
“Although ossification of articular cartilage from the underlying subchondral growth front is greatly diminished, it is not entirely stopped ”
” Because the fluid permeability of cartilage is quite low, it is difficult to squeeze the water out. Consequently, for short static loading periods or for cyclic loading with moderate or high frequencies, the tissue behaves mechanically as a single-phase solid. In these conditions, the simplest constitutive model represents cartilage as a homogeneous, linear elastic, incompressible or nearly incompressible material. ”
” Hydrostatic fluid pressure inhibits cartilage growth and ossification, thereby maintaining the cartilage phenotype. (ii) Tensile strain (or octahedral shear stress) accelerates cartilage growth, ossification and replacement by bone. (iii) Matrix compressive consolidation, with or without fluid pressure, decreases cartilage proteoglycan synthesis and content and results in a more fibrous cartilage phenotype.”
The vascularity and remodelling of subchondrial bone and calcified cartilage in adult human femoral and humeral heads. An age- and stress-related phenomenon.
“A quantitative study of the vascularity and a qualitative study of the remodelling of the calcified cartilage and subchondral bone end-plate of adult human femoral and humeral heads were performed with respect to age. In the femoral head the number of vessels per unit area was found to fall 20% from adolescence until the seventh decade and in the humeral head 15% until the sixth decade. Thereafter an increase was noted in the femur but none in the humerus. More vessels were present at all ages in the more loaded areas of the articular surfaces: 25% more for the femur and 15% more for the humerus. The degree of active remodelling by endochondral ossification declined 50% from adolescence until the seventh decade in the femoral head, and 30% until the sixth decade in the humeral head, rising thereafter to levels comparable to those found at young ages. More remodeling was noted in the more loaded areas at all ages.”
<-this study is cited in the above as one that’s showing that articular cartilage ossification occurs throughout life.