Gut microbiome is the microorganisms like bacteria that live in your digestive tract. The gut microbiome is influenced by your diet. There is a lot of research into the gut microbiome so if it does affect child’s height significantly improvements in that area could lead to dramatic increases in height for a population. Parent microbiome is passed on to a child so there is the possibility to increase height of a child by manipulating parent’s diet. Microbiome could impact height via IGF-1. Antiobiotic dosing which affects the gut microbiome has been shown to influence height. Changing the gut microbiome is not easy however. ” the composition of the gut microbiota is partially heritable and, once established, does not change substantially without a large or prolonged stimulus.“
Gut microbiota in regulation of childhood bone growth
“Longitudinal bone growth in children is governed by different genetic, nutritional and other environmental factors acting systemically on the endocrine system and locally at the growth plate. Recent studies have shown that this intricate interplay between nutritional and hormonal regulation of the growth plate could involve the gut microbiota, highlighting the importance of a holistic approach in tackling childhood undernutrition. In this review, I focus on the mechanistic insights provided by these recent advances in gut microbiota research and discuss ongoing development of microbiota-based therapeutics in humans, which could be the missing link in solving undernutrition and childhood stunting.”

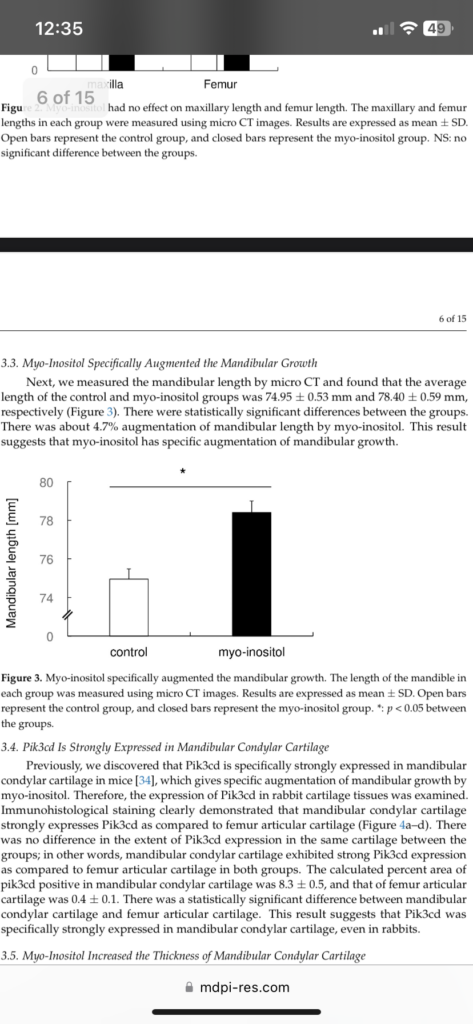
“Growth hormone stimulates production of IGF-I in the liver, which then acts as an endocrine factor to stimulate bone growth at the growth plate. Growth hormone also stimulates local IGF-I production in target tissues, such as the growth plate and the intestine, which acts as a paracrine/autocrine growth factor. Nutritional status positively regulates bone growth and maturation of the gut microbiota, which reciprocally promote nutritional intake. The gut microbiota also promotes bone growth, perhaps directly, by stimulating IGF-I production. Possible mechanisms for such stimulation might involve SCFAs and NOD2-mediated bacterial sensing pathways in the intestinal epithelial cells.”
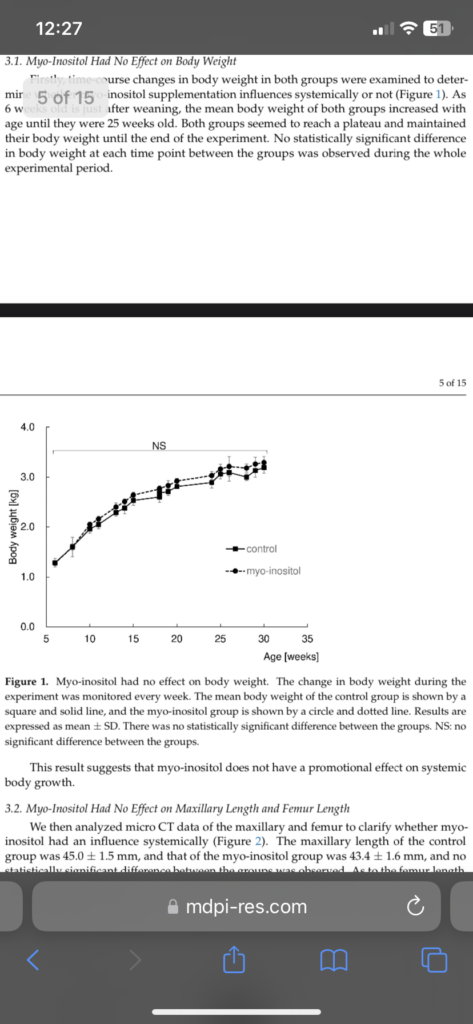
“growth deceleration is associated with the gradual decline in growth plate function, also known as growth plate senescence. Importantly, growth plate senescence is characterized by a gradual depletion of chondrogenic stem cells, decreasing chondrocyte proliferation and hypertrophy in the growth plate. Although growth plate senescence is generally associated with age, it appears not to be driven by age per se but instead depends on how much growth potential has been ‘used up’. In other words, chondrocytes in the growth plate appear to have a finite amount of growth potential, which is depleted gradually as more bone growth occurs, leading to the gradual decline in growth rate and the associated changes of senescence. This is supported by the fact that growth-inhibiting conditions, such as undernutrition, can slow down growth plate senescence, allowing bone growth not only to resume but temporarily to accelerate faster than normal for chronological age once nutritional status improves, a clinical phenomenon known as catch-up growth”<-so height seekers should have the goal to reverse senescence.
“The signal by which the gut microbiota stimulates IGF-I might not even be a metabolite. bacterial cell walls isolated from L. plantarum were sufficient to stimulate IGF-I and bone growth in mice”
“NOD2-activating ligands, such as muramyl dipeptide or the synthetic NOD2-activating adjuvant mifamurtide, alone were sufficient to induce IGF-I and bone growth, suggesting that NOD2 agonists could be a new class of therapeutic agents for improving childhood stunting.”
“another major cause of growth inhibition comes from a local effect of cytokines, which are often elevated in inflammatory diseases. At a systemic level, pro-inflammatory cytokines can inhibit bone growth by suppressing IGF-I. For example, in mice overexpressing interleukin-6 (IL-6), body growth is significantly suppressed, with decreased IGF-I and IGFBP3 but with normal levels of GH”
“The gut microbiota has been shown to influence circulating levels of pro-inflammatory cytokines. Serum IL-1β and IL-6 levels were correlated with the presence of certain bacterial strains in the gut microbiome. Mechanistically, butyrate, one of the SCFAs produced by the gut microbiota, has been shown to inhibit the inflammatory response elicit by lipopolysaccharides, TNFα and interleukins via GRP41 and GRP43, both in endothelial cells and in chondrocytes, suggesting that the gut microbiota could stimulate bone growth by reducing inflammation”

So you can use prebiotics and gut transplants potentially.
“In addition to the bare minimum of improving nutritional status, mitigation of gut microbiota dysbiosis, either by introducing growth-stimulating bacterial strains or by promoting gut microbiota maturation, should be considered as coupling therapeutic strategies.”
According to Fasting challenges human gut microbiome resilience and reduces Fusobacterium, fasting can alter the gut microbiome. Perhaps this could be how fasting affects bone length. “Water-only fasting could have a profound and long-lasting effect on gut microbiome.”
“Microbiome changes due to water-only fasting remained in five subjects even after returning to their normal diet, indicating the resilience of gut microbiome was successfully challenged. “