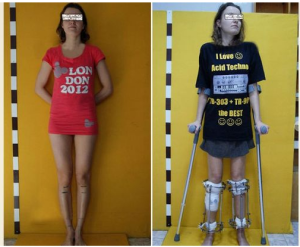
 This is sort of old news but it is sometimes still worth mentioning. Back in March of this year there was another story that came out of a person who got cosmetic limb lengthening surgery. Unlike most of the people you hear about, this person was a girl.
This is sort of old news but it is sometimes still worth mentioning. Back in March of this year there was another story that came out of a person who got cosmetic limb lengthening surgery. Unlike most of the people you hear about, this person was a girl.
While it is not very often we find girls who decide to go through with the surgery, there have been enough cases of girls who do it for the prospect of becoming a model. This girl fits that type of case study.
We refer to this article from Mirror, which is a UK based type media outlet/source. – http://www.mirror.co.uk/news/world-news/aspiring-model-spends-thousands-surgery-5115453
A 5′ 4″ tall aspiring model Alexandra Transer got the surgery done for a supposed 6,000 pounds and she has already gotten 6 cms out of it. She comes from a family of people who are below statured. Her mother is 5′ 2″ and her father is 5′ 5″. She chose not to go to University but work for her father in Engineering and saved up the 6,000 UK pounds to get the surgery.
She had wanted to be a model for most of her life, but when she contacted a modeling agency, they told her that she was below the height requirement and that the only way for her to get to that strict modeling height requirement was to extend her legs. She decided to do just that and went through with it, which we found very impressive and brave.
This first surgery she has decided to do is one of a total of 3 planned. There is supposed to be 2 more surgeries she will go through which will eventually put her at 6 feet. Her estimated time frame is that the 3rd surgery will be done by 2018. She will be in her 30s by then and may not get any type of modeling work but she did say that she plans to dye her hair blonde and then feel like a model being tall and blonde.
It seems that maybe what she really wants is to feel beautiful. On this website, we don’t judge. Vanity is something we all go through.
Some new information we learned is that she is actually from Russia herself, Volgograd. For anyone familiar with the LLS community, Volgograd is actually really famous because of the Centre of Anthropometrical (Orthopaedical) Cosmetology and Correction. Refer to Dr. Alexander Barinov and Dr. Viktor Shatov, of the center RUCOSM. Dr. Alexander Barinov is famous for being a protege and student to the more legendary Dr. Yegorov.
She mentioned that a former Soviet Athlete, a Valery Brumel, got this type of surgery done on them in 1968. Why did this olympic athlete get it done back in the 60s? We don’t know about that. I personally suspect that one of the reasons she was willing to go through with the surgery unlike so many other people who wish to be taller but are too afraid of surgery is because she comes from Russia, and specifically her home town has one of the most popular and recognizable centers for height increase surgery in the world. I am sure her parents at first was very reserved in her desires but they probably had a better understanding for why she would go through with it than other parents. Since they are from Russia originally, going a center like RUCOSM would not be as difficult of a transition as other people from other countries who might know any of the local Russian customs or language. Maybe for this girl, going to get limb lengthening surgery is as simple as driving the 3 miles to one’s local gym center to exercise.
Based on current conversion rates, it seems like she paid about $9,400 USD to get the first surgery.
We here have done our own level of research on LLS, and the cost of it. Most people have said that the cost of getting LLS is usually about $50,000 with a trip to one of the more well known, reputable surgeons costing upwards of $100,000 with everything covered, for maybe 10 cm or 4 Inches of increase in the leg bones. The lowest cost I personally have heard of was around $10,000 for the cost of surgery from a former Chinese surgeon. The reduced price of getting one’s leg extended seems to suggest that the cosmetic procedure may be becoming much more prevalant. That may be a good thing for some people who really want to go through with it and are looking for a cheaper alternative.
When we looked at the cost for LLS back in 2011, it seems that the cost was around 12,000 Euros, at least in the RUCOSM center, in Volgograd. Our source was from the Short Support Group website. The 12,000 euros cost was supposed to cover pretty much everything, including a 3 month stay. Of course, from the RUCOSM website, they state that for each surgery, they can only increase one’s bone length by 7-9 cm maximum. This is why Alexandra needed 3 total surgeries, to gain 8 inches or 20 cms of height to reach her desired goal of 6 feet tall from her original 5′ 4″. For her, she was very happy with the results, and there were no complications.
Refer to here for an interview with Dr. Barinov.
Note what he claimed for the cost of the surgery
- How much does the height increasing procedure cost? – Dr.Yegorov works with different clinics, both state and private. The total cost of the procedure including all necessary expenses is from $15,000 to $30,000 US dollars depending on the clinic and the accommodation and service conditions.
We would say that $10,000 USD is a little unrealistic of cost. A more reasonable cost would be probably $25,000 and that would just be for 7-8 cms of increase, assuming that no complications are developed. Of course those will be 2011 prices. Since it is 2015, We should expect maybe $30-35 K in fees in total. How Alexandra was able to just pay $10,000 we are not sure. Maybe she got her surgery done in the UK, and not Russia. Maybe instead of paying the thousands of dollars in fee for staying at the hospital facility to recuperate, she was able to have someone take her to her home in Volgograd (which might just be a few blocks away) during that time, allowing her to save thousands in hospital fees.