I actually missed that this LSJL study showed height increase as it was a minor comment that joint loading increased height of the femoral head. Since the femoral head is a diagonal offshoot of the femur it may not necessarily increase height but it is an increase in bone length all the same. However, the osteonecrosis induced in the study may be a prerequisite to induce the femoral head growth. However, it should be noted that the osteonecrosis decreased femoral head height but the degradation of existing bone may have allowed for new bone growth.
This is an LSJL related study published by Yokota and Zhang. The primary author seems to be more Zhang who seems more into the lengthening effects than Yokota. This study shows that joint loading increases fibrous differentiation. And fibrous differentiation would be a potential intermediary step for neo-growth plate formation. Knee loading increased vessel remodeling and osteoclast formation which would be needed to make room for a new growth plate but these levels were only slightly greater than control group and reduced from the osteonecrosis group so we can not say for sure whether this will happen in LSJL on a normal bone.
Overall this study shows that LSJL does at least one of the steps required for neo-growth plate formation: fibrous type tissue formation. This fibrous tissue would then have to be further differentiated into more cartilagenous tissue. The perichondrial ring(or ring of LaCroix) is fibrocartilagenous.
Knee loading protects against osteonecrosis of the femoral head
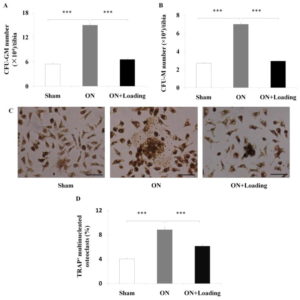
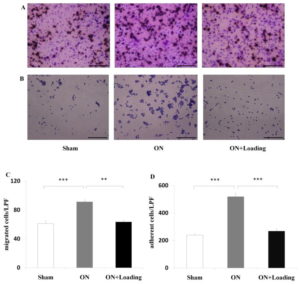
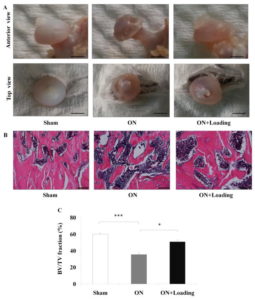
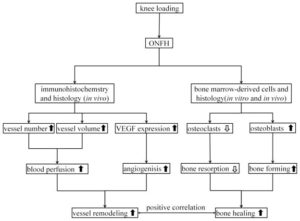
“Osteonecrosis[loss of blood to the bone] of the femoral head is a serious orthopedic problem. Moderate loads with knee loading promote bone formation. Using a rat model, we examined a hypothesis that knee loading enhances vessel remodeling and bone healing through the modulation of the fate of bone marrow-derived cells. In this study, osteonecrosis was induced by transecting the ligamentum teres followed by a tight ligature around the femoral neck. For knee loading, 5 N loads were laterally applied to the knee at 15 Hz{this is pretty high frequency} for 5 min/day for 5 weeks. Changes in bone mineral density (BMD) and bone mineral content (BMC) of the femur were measured to evaluate vessel remodeling. Femoral heads were harvested, and bone marrow-derived cells were isolated to examine osteoclast development and osteoblast differentiation. Osteonecrosis significantly induced bone loss, and knee loading stimulated both vessel remodeling{vessel remodeling shows promise as that would be very helpful for neo growth plate creation} and bone healing. The osteonecrosis group exhibited the lowest trabecular BV/TV in the femoral head, and lowest femoral BMD and BMC. Knee loading increased trabecular BV/TV as well as BMD and BMC. Osteonecrosis decreased the vessel volume, vessel number and VEGF expression, and knee loading increased them. Osteonecrosis activated osteoclast development, and knee loading reduced its formation, migration, adhesion and the level of “pit” formation{pit formation could potentially be beneficial though as that pit could where a neo-growth plate would go}. knee loading significantly increased osteoblast differentiation and CFU-F{An increase in CFU-F means an increase in mesenchymal stem cell proliferation which is a good for neo-growth plate formation but doesn’t guarantee that it will occur}. A significantly positive correlation was observed between vessel remodeling and bone healing. Knee loading could be effective in repair osteonecrosis of the femoral head in a rat model [by] promoting vessel remodeling, suppressing osteoclast development, and increasing osteoblast and fibroblast differentiation. “
“The mechanism of knee loading is considered to change intramedullary pressure of femoral and tibial bone cavities. The load driven pressure may generate fluid flow in a lacuna canalicular network in bone cortex. The pressure activates bone metabolism-related genes in femur and tibia”<-what we are interested in an increase in fluid flow and hydrostatic pressure to induce chondrogenic differentiation. Hydrostatic pressure by itself isn’t likely to induce chondrogenic differentiation by itself as it is typically three orders of magnitude below the pressure needed to induce chondrogenic differentiation. But the addition of bone deformation and fluid flow may bridge the gap and induce chondroinduction.
“Male Sprague–Dawley rats (~12weeks of age)”
“knee loading was achieved through dynamic loads applied to the left and right knee joints of rats in the lateral–medial direction, respectively. To position the knee properly, the lower end of the loading rod and the upper end of the stator were designed to form a pair of semispherical cups. The lateral and medial epicondyles of the femur together with the lateral and medial condyles of the tibia were confined in the cups. The tip of the loader had a contact
area of 15 mm in diameter.”
See Figure 1 of the paper(first link on the page) for an image of the knee loader.
Loading increased vascular remodeling in the osteonecrosis + LSJL group but not versus control. But there was no normal bone plus loading group.
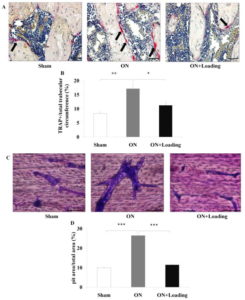
Joint Loading actually seemed to reduce the number of mesenchymal stem cells but that could be due an increase in differentiation. Mesenchymal condensation was more visible in the osteonecrosis group and mesenchymal condensation is a prerequisite for neo-growth plate formation.
Knee Loading seemed to restore osteoclast adhesion and migration to slightly above control levels but that slightly above could indicate activity that would remodel the bone enough to allow for mesenchymal condensation.
Knee loading greatly increased CFU-F and Osteoblast differentiation versus both osteonecrosis and control group. “Knee loading enhanced differentiation of osteoblasts and fibroblasts.” Fibroblastic tissue(marked by CFU-F) could potentially become chondrogenic tissue. Fibrocartilage is a thing. But to be a true growth plate, additional mechanical stimulation would be needed to turn that fibrocartilage into pure cartilage.
” Knee loading increased the height of the femoral head partially”
It’s a big breakthrough that LSJL increased height in the femoral head. The osteonecrotic bone had far more marrow in B, thus under osteonecrosis LSJL may have had more room to induce growth.
The perichondrial ring of the growth plate is fibrocartilagenous in nature and may be the source to provide cells to the growth plate.
“The current histology and bone mineral density data are suggestive of the role of bone marrow-derived stem cells in load-driven bone healing, and they also establish a causal relationship between the observed vessel remodeling and bone healing effects with knee loading”<-This is huge because we want to induce stem cells to form neo-growth plates.
According to this LSJL would increase blood perfusion which would be the most interesting thing. If the bone has more blood flowing through can create unique biological opportunities remember one of the key steps to distraction osteogenesis is a blood clot.
It was mentioned that LSJL upregulates bone metabolism related genes. Here’s a study that lists bone metabolism related genes:
“bone metabolism-related markers, such as serum concentrations of calcium, phosphorus, PTH,
and 25(OH) D”
“genetic variants of MEF2C, ESR1, TNFRSF11B, and SOX6 were associated with bone metabolism-related markers”