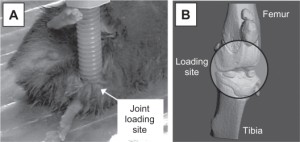
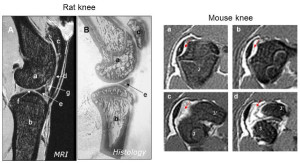
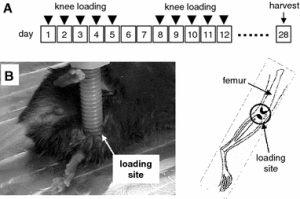
Loading bones against each other also loads the ligaments against the growth plate. One method of LSJL involves pushing bones against each other at the points of the epiphysis.
Mesenchymal stem cell characteristics of human anterior cruciate ligament outgrowth cells.
“When ruptured, the anterior cruciate ligament (ACL) of the human knee has limited regenerative potential. Cells that migrate out of the human ACL constitute a rich population of progenitor cells and we hypothesize that they display mesenchymal stem cell (MSC) characteristics when compared with adherent cells derived from bone marrow or collagenase digests from ACL. ACL outgrowth cells are adherent, fibroblastic cells with a surface immunophenotype strongly positive for cluster of differentiation (CD)29, CD44, CD49c, CD73, CD90, CD97, CD105, CD146, and CD166, weakly positive for CD106 and CD14, but negative for CD11c, CD31, CD34, CD40, CD45, CD53, CD74, CD133, CD144, and CD163. Staining for STRO-1 was seen by immunohistochemistry but not flow cytometry. Under suitable culture conditions, the ACL outgrowth-derived MSCs differentiated into chondrocytes, osteoblasts, and adipocytes and showed capacity to self-renew in an in vitro assay of ligamentogenesis. MSCs derived from collagenase digests of ACL tissue and human bone marrow were analyzed in parallel and displayed similar, but not identical, properties. In situ staining of the ACL suggests that the MSCs reside both aligned with the collagenous matrix of the ligament and adjacent to small blood vessels. The cells that emigrate from damaged ACLs are MSCs have the potential to provide the basis for a superior, biological repair of this ligament.”
“mobile population of MSCs within the ACL”
“cells derived from both ACL sources and bone marrow underwent chondrogenic differentiation in the presence, but not absence, of TGF-β1”
“MSCs migrate to sites of injury”
So ligaments near the epiphysis can differentiate into chondrocytes.
Chondrocyte phenotype and ectopic ossification in collagenase-induced tendon degeneration.
“Chondrocyte phenotype and ectopic ossification in a collagenase-induced patellar tendon{in the new LSJL model the patella is a targetfor loading} injury model. Collagenase or saline was injected intratendinously in one limb. The patella tendon was harvested for assessment at different times. There was an increase in cellularity, vascularity, and loss of matrix organization with time after collagenase injection. The tendon did not heal histologically until week 32. Ectopic mineralization started from week 8. Tendon calcification was mediated by endochondral ossification, as shown by expression of type X collagen. viva CT imaging and polarization microscopy showed characteristic bony porous structures and collagen fiber arrangement, respectively, in the calcific regions. Marrow-like cells and blood vessels were observed inside calcific deposits. Chondrocyte-like cells as indicated by morphology, expression of type II collagen, and sox 9 were seen around and embedded inside the calcific deposits. Fibroblast-like cells expressed type II collagen and sox 9 at earlier times, suggesting that erroneous differentiation of healing tendon fibroblasts may account for failed healing and ossification in collagenase-induced tendon degeneration.”
“Chondrocyte markers were expressed in the clinical samples of calcific insertional Achilles tendinopathy”
“Chondrocyte-like cells as indicated by cellular morphology and expression of sox 9 and type II collagen were observed around the calcific deposits in collagenase-induced degenerative tendon injury”
“The differentiation of tendon progenitor cells into chondrocytes and bone cells was reported to be modulated by the expression of small leucine-rich repeat proteoglycans such as biglycan and fibromodulin, which control the differentiation process associated with BMP-2 activities ”
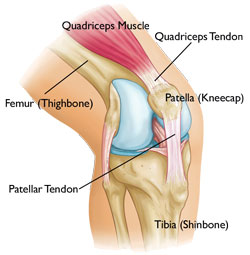
The tendons are relatively close to the epiphysis as well.
The origin points of the knee collateral ligaments: an MRI study on paediatric patients during growth
“Different femoral origins for both the medial collateral ligament (MCL) and the lateral collateral ligament (LCL) have been reported in the growing skeleton (epiphyseal and metaphyseal). This study assesses the femoral origins of the knee collateral ligaments in skeletally immature individuals.
MRIs of 336 knee joints (median age 15 years (range 2–18 years)) were retrospectively analysed to assess the distances between the femoral origins of the MCL and LCL to the distal femoral growth plate.
Both MCL and LCL ligament origins were invariably located on the epiphysis. Mean MCL origin–growth plate distance was 9.6 mm (SD 2.1 mm; range 2.2–13.6 mm) in boys and 8.6 mm (SD 1.5 mm; range 3.4–12.0 mm) in girls{The MCL ligament is very close to the growth plate}. Mean LCL origin–growth plate distance was 9.3 mm (SD 1.8 mm; range 4.3–13.0 mm) in boys and 8.2 mm (SD 1.5 mm; range 3.4–11.8 mm) in girls{The LCL ligament is very close to the growth plate}. The distance between the growth plate and both collateral ligaments as well as the length of the LCL correlated positively with patients’ age and body size.
During growth, the femoral origins of the MCL and the LCL are constantly located on the distal femoral epiphysis. There is a linear increase in the distances from the ligaments’ origins to the growth plate according to age and body size.”
“The LCL attaches slightly closer to the growth plate than the MCL. The distances between the origins of the ligaments and the distal femoral growth plate increase in a nearly linear pattern until closure of the growth plate.”
“Entheses (insertion sites, osteotendinous junctions, osteoligamentous junctions) are sites of stress concentration at the region where tendons and ligaments attach to bone.”
“Tendons and ligaments can be regarded as machines with multiple moving parts (fibrils, fibres and fascicles) that perform the basic function of force transfer to and from the skeleton. They distribute the loads applied to them dynamically in order to execute movement patterns. Their complex response to loading allows for multi-axis bending, and this adds to the stress concentration in the region where they attach to bone. This attachment site will be referred to in this review as an ‘enthesis’, but it is also known as an ‘insertion site’, or an ‘osteotendinous’ or ‘osteoligamentous’ junction.”
The tissue at the enthesis insertion site is either fibrous or fibrocartilagenous.
“chondral–apophyseal entheses are found at the ends of the long bones and periosteal–diaphyseal attachments occur on the shafts.”
“At fibrous entheses, the tendon or ligament attaches either directly to the bone or indirectly to it via the periosteum. In both cases, dense fibrous connective tissue connects the tendon/ligament to the periosteum and there is no evidence of (fibro)cartilage differentiation”
“Fibrocartilaginous entheses are sites where chondrogenesis has occurred and thus four zones of tissue are commonly present: pure dense fibrous connective tissue, uncalcified fibrocartilage, calcified fibrocartilage and bone”
“The inclusion of a zone of ‘pure dense fibrous connective tissue’ and a zone of ‘bone’ at a fibrocartilaginous enthesis highlight the difficulty of defining with any degree of precision where such an enthesis begins and ends.”<-Thus the ligament that attatches to the epiphysis near the growth plate line may extend partially into the bone itself.
“the proportion of the enthesis subchondral bone plate which consists of calcified fibrocartilage increases with age, because of a thinning of the cortical bone”
“Although tendons and ligaments are often viewed as non-distensible, they do have the ability to stretch and recoil by approximately 6% of their original length without any obvious signs of damage.”
“entheses can act as growth plates for apophyses at tendon and ligament attachment sites.”
“cartilage at the enthesis is initially derived from that of the embryonic bone rudiment.”
“this hyaline cartilage is eroded during endochondral ossification and replaced by enthesis fibrocartilage that develops within the adjacent ligament by fibroblast metaplasia.”
“Bony spurs (enthesophytes) are well documented at numerous entheses as bony outgrowths that extend from the skeleton into the soft tissue of a tendon or ligament at its enthesis”<-our goal is a cartilage ingrowth into the bone from the tendon/ligament.
Magnetic resonance imaging of entheses. Part 1.
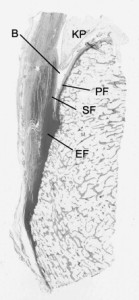
“Achilles tendon, sagittal, histological section stained with Toluidine blue. Enthesis (EF) sesamoid (SF) and periosteal (PF) fibrocartilages are seen. The retrocalcaneal bursa (B) lies between the tendon and the bone and contains the tip of Kager’s retromalleolar fat pad (KP).”
“One of the interesting features of fibrocartilaginous entheses is the paucity[scarcity] of compact bone (often referred to as the cortical shell) immediately beneath the attachment site. The subchondral plate (i.e. the associated calcified fibrocartilage and cortical shell) is often very thin and in many fibrocartilaginous entheses, there are local areas where subchondral bone and calcified fibrocartilage are absent.“<-Thus, it would be easier for cells to migrate there.
“Fibrocartilage forms in tendons that are translocated around bony pulleys and regresses in tendons that are re-routed so that they are no longer subject to compression in the same region.”