When I was doing my research to learn more about the detail of the growth plates and it’s subsections, I read that the chondrocytes creates a waste or output which is collagen and proteoglycan. I have already looked at the effects and possibilities of Collagen Type II on height and height increase in a previous article. Now in this post, I wanted to explore the affect and effects that proteoglycan has on human growth and height.
First, What is proteoglycans?
From the Medical Dictionary website (source HERE)…
proteoglycan /pro·teo·gly·can/ (pro″te-o-gli´kan) any of a group of polysaccharide-protein conjugates present in connective tissue and cartilage, consisting of a polypeptide backbone to which many glycosaminoglycan chains are covalently linked; they form the ground substance in the extracellular matrix of connective tissue and also have lubricant and support functions.
pro·te·o·gly·can (pr  t t – – -gl -gl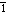 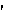 k k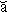 n n , -k , -k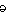 n) n)
n. Any of various mucopolysaccharides that are bound to protein chains in covalent complexes and occur in the extracellular matrix of connective tissue.
|
proteoglycan [pro″te-o-gli´kan]
——->
For a further in depth detail, From Wikipedia (source HERE)…
Proteoglycans are proteins that are heavily glycosylated. The basic proteoglycan unit consists of a “core protein” with one or more covalently attachedglycosaminoglycan (GAG) chain(s). The point of attachment is a Ser residue to which the glycosaminoglycan is joined through a tetrasaccharide bridge (For example: chondroitin sulfate-GlcA-Gal-Gal-Xyl-PROTEIN). The Ser residue is generally in the sequence -Ser-Gly-X-Gly- (where X can be any amino acid residue), although not every protein with this sequence has an attached glycosaminoglycan. The chains are long, linear carbohydrate polymers that are negatively charged under physiological conditions, due to the occurrence of sulfate and uronic acid groups. Proteoglycans occur in the connective tissue.
Proteoglycans can be categorised depending upon the nature of their glycosaminoglycan chains. Proteoglycans can also be categorised by size (kDa).
Types
Types include:
| Glycosaminoglycans | Small proteoglycans | Large proteoglycans |
|---|---|---|
| chondroitin sulfate/dermatan sulfate | decorin, kDa=36
biglycan, kDa=38 |
versican, kDa=260-370, present in many adult tissues including blood vessels and skin |
| heparan sulfate/chondroitin sulfate | testican, kDa=44 | perlecan, kDa=400-470 |
| chondroitin sulfate | neurocan, kDa=136
aggrecan, kDa=220, the major proteoglycan in cartilage |
|
| keratan sulfate | fibromodulin, kDa=42
lumican, kDa=38 |
Certain members are considered members of the “small leucine-rich proteoglycan family” (SLRP).[3] These include decorin, biglycan, fibromodulin and lumican.
Function
Proteoglycans are a major component of the animal extracellular matrix, the “filler” substance existing between cells in an organism. Here they form large complexes, both to other proteoglycans, tohyaluronan and to fibrous matrix proteins (such as collagen). They are also involved in binding cations (such as sodium, potassium and calcium) and water, and also regulating the movement of molecules through the matrix. Evidence also shows they can affect the activity and stability of proteins and signalling molecules within the matrix. Individual functions of proteoglycans can be attributed to either the protein core or the attached GAG chain and serve as lubricants.
Synthesis
The protein component of proteoglycans is synthesized by ribosomes and translocated into the lumen of the rough endoplasmic reticulum. Glycosylation of the proteoglycan occurs in the Golgi apparatus in multiple enzymatic steps. First a special link tetrasaccharide is attached to a serine side chain on the core protein to serve as a primer for polysaccharide growth. Then sugars are added one at a time by glycosyl transferase. The completed proteoglycan is then exported in secretory vesicles to the extracellular matrix of the cell.
Proteoglycans and disease
An inability to break down proteoglycans is characteristic of a group of genetic disorders, called mucopolysaccharidoses. The inactivity of specific lysosomal enzymes that normally degrade glycosaminoglycans leads to the accumulation of proteoglycans within cells. This leads to a variety of disease symptoms, depending upon the type of proteoglycan that is not degraded.
ME: What is important to takeaway from this is that Proteoglycans (mucoproteins) are formed of glycosaminoglycans (GAGs) covalently attached to the core proteins. There are many types of glycosaminoglycans (GAGs). The most commonly well known GAGs are Hyaluronic acid, Dermatan sulfate, Chondroitin sulfate, Heparin and heparin sulfate, and Keratan sulfate (source HERE)
From source http://www.cryst.bbk.ac.uk/pps97/assignments/projects/emilia/Proteoglycans.HTM
Structure of proteoglycans
The GAGs extend perpendicular from the core protein in a bottlebrush- like structure.
The linkage of GAGs such as (heparan sulfates and chondroitin sulfates) to the protein core involves a specific trisaccharide linker :
The protein cores of proteoglycans are rich in Ser and Thr residues which allows multiple GAG attachment.
Role of proteoglycans and glycosaminoglycans
They perform numerous vital functions within the body.
GAG dependent functions can be divided into two classes: the biophysical and the biochemical.
The biophysical functions depend on the unique properties of GAGs : the ability to fill the space, bind and organize water molecules and repel negatively charged molecules. Because of high viscosity and low compressibility they are ideal for a lubricating fluid in the joints. On the other hand their rigidity provides structural integrity to the cells and allows the cell migration due to providing the passageways between cells.
For example the large quantities of chondroitin sulfate and keratan sulfate found on aggrecan play an important role in the hydration of cartilage. They give the cartilage its gel-like properties and resistance to deformation.
Aggrecan is one of the most important extracellular proteoglycans. It forms very large aggregates (a single aggregate is one of the largest macromolecules known ; it can be more than 4 microns long). Aggrecan molecules are non-covalently bound to the long molecule of hyaluronan (like bristles to the backbone in a bottlebrush). It is faciliated by the linking proteins. To each aggrecan core protein multiple chains of chondroitin sulfate and keratan sulfate are covalently attached through the trisaccharide linker .
The other, more biochemical functions of GAGs are mediated by specific binding of GAGs to other macromolecules, mostly proteins. Proteoglycans participate in cell and tissue development and physiology.
EXAMPLES OF GAG BINDING PROTEINS :
Secreted proteases and antiproteases
For example antithrombin III (AT III) binds tightly to heparin and certain heparan sulfates (so do its substrates). Thus they control the blood coagulation. In the absence of GAGs AT III inactivates proteases (such asthrombin, factors IXa and XIa) very slowly. In the presence of appropriate GAGs these reactions are accelerated 2000-fold.
GAGs are sufficiently long that both protease and protease inhibitor can bind to the same chain (thus the likelyhood of the two proteins binding to each other is increased enormously). GAGs also affect the protein conformation that contributes to improving AT III binding kinetics.
Polypeptide growth factors
Members of the FGF family, as well as several other growth factors, bind to heparin or heparan sulfate. Binding to endogenous GAGs entraps these molecules in ECM from which they may be later released. GAGs can alter the conformation, proteolytic susceptibility and biological activity of some of these proteins. The bound growth factor is resistant to degradation by extracellular proteases. Active hormone is released by proteolysis of the heparan sulfate chains. It occurs during the tissue growth and remodeling after infection.

Pingback: Complete List Of Posts - |
Pingback: The Connection Between Aggrecan, Chondrogenesis, And Height - Natural Height Growth | Natural Height Growth
Pingback: Spinal height gained by bedrest could be related to GAGs - Natural Height Growth