At some point in my research, I realized that one critical pathway I had to familiarize myself with was a signaling pathway called the AKT signaling pathway. This post is my attempt at understanding it and how it is related to human growth. One thing to note is that it seems the AKT pathway is also named the PI3K pathway but the PI3K is different from the AKT although the two parts are connected..
It turns out that this pathway is quite complex. A simpler way for the reader is just to watch this YouTube video HERE.
Note: A Lot of the information and material I will write up below will be also be transferred and added to the section for “Protein/Hormone Pathway Map” post/section as well as the “Molecular Biology, Biochemistry” section. As always, any section and sub-section of the website will always be edited and added upon as time goes on.
From source 1, it seems that “PI3K activation activates AKT which activates mTOR.” As we remember mTOR has been shown to be involved in possible height increase since its inhibitor rapamycin also destroys leucine which is shown to play a role in growth.
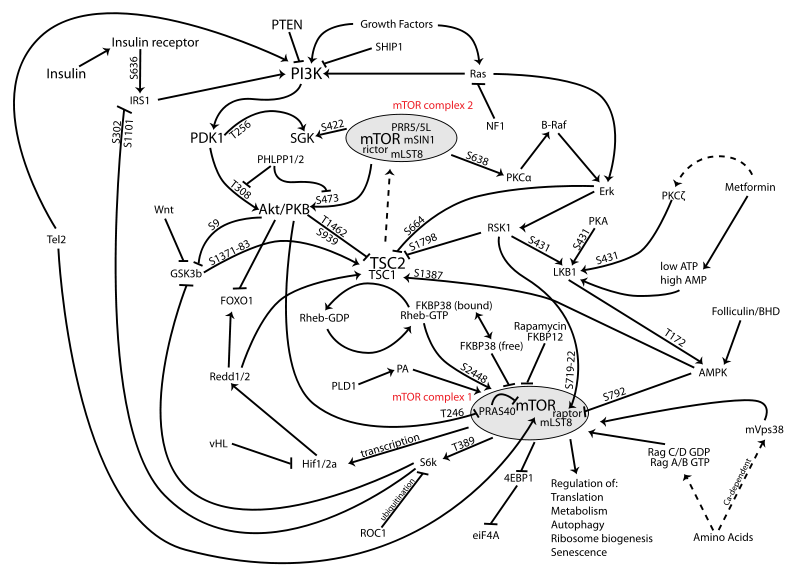
From source 2, I post a picture of the mTOR/AKT/PI3K pathway below. For this diagram, the arrows seem to mean either you are inhibiting something or promoting it.
The two major parts of the pathway is are the PI3Ks and the AKTs
The type of pathway shows what happens from the cell’s cytoplasm to the cell’s nucleus. In the nucleus you have three types of information transformation, transcription, reverse transcription, and translation.
From the Wikipedia article on mTOR inhibitors…
The mammalian target of rapamycin (mTOR) also known as mechanistic target of rapamycin or FK506 binding protein 12-rapamycin associated protein 1 (FRAP1) is a protein which in humans is encoded by the FRAP1 gene.
Function
mTOR is a serine/threonine protein kinase that regulates cell growth, cell proliferation, cell motility, cell survival, protein synthesis, and transcription. mTOR belongs to the phosphatidylinositol 3-kinase-related kinase protein family.
mTOR integrates the input from upstream pathways, including insulin, growth factors (such as IGF-1 and IGF-2), and amino acids. mTOR also senses cellular nutrient and energy levels and redox status. The mTOR pathway is dysregulated in human diseases, especially certain cancers, Rapamycin is a bacterial product that can inhibit mTOR by associating with its intracellular receptor FKBP12. The FKBP12 rapamycin complex binds directly to the FKBP12-Rapamycin Binding (FRB) domain of mTOR.
mTOR is the catalytic subunit of two molecular complexes.
mTOR stands for mammalian Target Of Rapamycin and was named based on the precedent that TOR was first discovered via genetic and molecular studies of rapamycin-resistant mutants of Saccharomyces cerevisiae that identified FKBP12, Tor1, and Tor2 as the targets of rapamycin and provided robust support that the FKBP12-rapamycin complex binds to and inhibits the cellular functions of Tor1 and Tor2.
Analysis & Interpretation:
It would seem that the PI3K/AKT/mTOR pathway is one of the most important pathways in the intracellular system (within the cytoplasm). The mTOR is the target protein (mammalian target and mechanistic target) of something else called a Rapamycin. The other name for this protein is FK506 binding protein 12-rapamycin associated protein 1 (FRAP1). mTOR has the big job of regulating growth, cell proliferation, survical, protein synthesis, and transcription. The thing is that when the IGF-1, IGF-2, insulin which are growth factors come to the body, they are combined together by this protein. This shows that it is a sort of a middle of the process and critical element determining the effectiveness of the growth factors we get in our bodies. It also has the ability to sense cell nutrient and energy levels. When there is a type of diseases, the pathway’s ability to regulate stuff gets disrupted like in cancer. The way that rapamycin blocks the mTOR is to get itself attached to the mTOR receptor, something called FKBP12. When the FKBP12 is connected the the rapamycin, the complex that is formed inhibits the function of Tor1 and Tor2.
From the Wikipedia article on the Serine/Threonine Protein Kinase ,
A serine/threonine protein kinase is a kinase enzyme that phosphorylates the OH group of serine or threonine (which have similar sidechains). At least 125 of the 500+ human protein kinases are serine/threonine kinases (STK)….Serine/Threonine Kinase receptors plays a role in the regulation of cell proliferation, programmed cell death (apoptosis), cell differentiation, and embryonic development.
Analysis & Interpretation:
What we learn about a serine and/or threonine protein kinase is that it is a type of enzyme that takes off the -OH ends of the serine and threonine amino acids and replaces the stub where the -OH group used to be with a phosphate group, a PO4(3-). It seems that there is a bigger group known as protein kinases. Within this bigger group of protein kinases, the serine/threonine kinases are the subset but represent a huge percentage of the number of protein kinases in the human body. We learn that the receptors for the serine and threonine protein kinases have some sort of role in the regulation of cell proliferation, in apoptosis, cell differentiation, and embryonic development. It is very clear from this small section that the serine/threonine protein kinases as a group is very important in determining the growth and development of the cells and the human body. Since the mTOR is a type of protein kinase, it would be important as well.
From the Wikipedia article on Kinase…
In biochemistry, a kinase is a type of enzyme that transfers phosphate groups from high-energy donor molecules, such as ATP, to specific substrates, a process referred to as phosphorylation. Kinases are part of the larger family of phosphotransferases. Kinases are not to be confused with phosphorylases, which carry out phosphorolysis, the breaking of a bond using an inorganic phosphate group; or with phosphatases, which remove phosphate groups.
Types
One of the largest groups of kinases are protein kinases, which act on and modify the activity of specific proteins. Kinases are used extensively to transmit signals and control complex processes in cells. More than five hundred different kinases have been identified in humans. Their enormous diversity, as well as their role in signaling, makes them an object of study.
Various other kinases act on small molecules such as lipids, carbohydrates, amino acids, and nucleotides, either for signaling or to prime them for metabolic pathways. Kinases are often named after their substrates.
Analysis & Interpretation:
We get another definition of what the term “phosphorylation” actually means. Phosphorylation is where a molecule in a state of high energy donates (transfers) a phosphate group (PO4(3-)) to a specific substrate. An example of a high energy donor molecule would be ATP (Adenosine triphosphate). Protein Kinases specfically change the activity of specific proteins. Kinases in general are used a lot to transmit signals and complex processes in cells.
From the Wikipedia article on Protein Kinase…
A protein kinase is a kinase enzyme that modifies other proteins by chemically adding phosphate groups to them (phosphorylation). Phosphorylation usually results in a functional change of the target protein (substrate) by changing enzyme activity, cellular location, or association with other proteins. The human genome contains about 500 protein kinase genes and they constitute about 2% of all human genes. Protein kinases are also found in bacteria and plants. Up to 30% of all human proteins may be modified by kinase activity, and kinases are known to regulate the majority of cellular pathways, especially those involved in signal transduction.
The chemical activity of a kinase involves transferring a phosphate group from a nucleoside triphosphate (usually ATP) and covalently attaching it to specific amino acidswith a free hydroxyl group. Most kinases act on both serine and threonine (serine/threonine kinases), others act on tyrosine (tyrosine kinases), and a number act on all three (dual-specificity kinases). There are also protein kinases that phosphorylate other amino acids, including histidine kinases that phosphorylate histidine residues.
Regulation
Because protein kinases have profound effects on a cell, their activity is highly regulated. Kinases are turned on or off by phosphorylation (sometimes by the kinase itself – cis-phosphorylation/autophosphorylation), by binding of activator proteins or inhibitor proteins, or small molecules, or by controlling their location in the cell relative to their substrates.
Analysis & Interpretation:
Protein kinase is an enzyme (aka protein which chances the function of other proteins by adding a molecular unit of a phosphate group to them. This process of adding a phosphate group is called phosphorylation. The protein that gets a phosphate group added on is known as the target protein as well as the substrate. Their activity, location, and way they interact with other proteins all get changed. There is around 500 protein kinases and they make up around 2% of the all the genes in the genome. About 30% of all human proteins can be changed by the function of the kinases. One of the functions of the kinases is to regulate the pathways in the cells, but most especially the ones that are involved in sinal transduction.
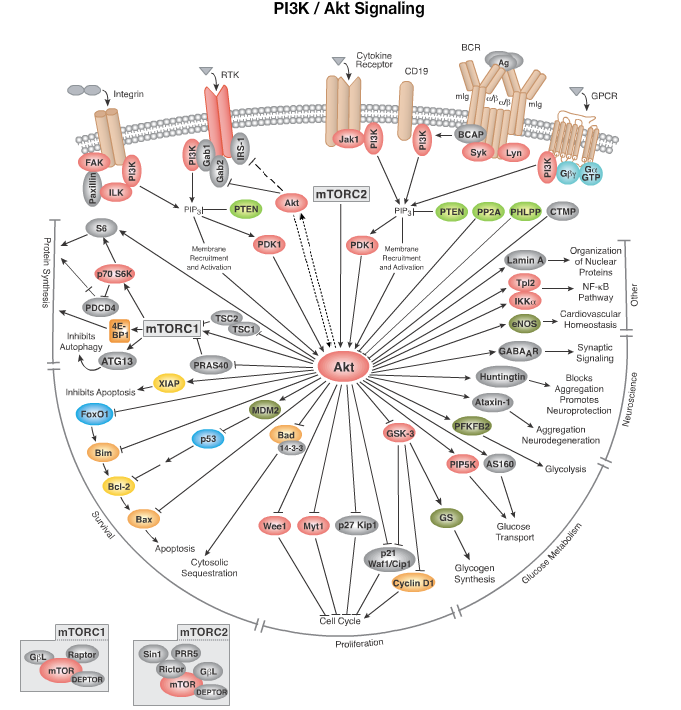
From the website CellSignal.com, I post a picture below that I found from the website.
Pathway Description:
Since its initial discovery as a proto-oncogene, the serine/threonine kinase Akt (also known as protein kinase B or PKB) has become a major focus of attention because of its critical regulatory role in diverse cellular processes, including cancer progression and insulin metabolism. The Akt cascade is activated by receptor tyrosine kinases, integrins, B and T cell receptors, cytokine receptors, G protein coupled receptors and other stimuli that induce the production of phosphatidylinositol 3,4,5 triphosphates (PtdIns(3,4,5)P3) by phosphoinositide 3-kinase (PI3K). These lipids serve as plasma membrane docking sites for proteins that harbor pleckstrin-homology (PH) domains, including Akt and its upstream activator PDK1. There are three highly related isoforms of Akt (Akt1, Akt2, and Akt3) and these represent the major signaling arm of PI3K. For example, Akt is important for insulin signaling and glucose metabolism, with genetic studies in mice revealing a central role for Akt2 in these processes. Akt regulates cell growth through its effects on the mTOR and p70 S6 kinase pathways, as well as cell cycle and cell proliferation through its direct action on the CDK inhibitors p21 and p27, and its indirect effect on the levels of cyclin D1 and p53. Akt is a major mediator of cell survival through direct inhibition of pro-apoptotic signals such as Bad and the Forkhead family of transcription factors. T lymphocyte trafficking to lymphoid tissues is controlled by the expression of adhesion factors downstream of Akt. In addition, Akt has been shown to regulate proteins involved in neuronal function including GABA receptor, ataxin-1, and huntingtin proteins. Akt has been demonstrated to interact with Smad molecules to regulate TGFβ signaling. Finally, lamin A phosphorylation by Akt could play a role in the structural organization of nuclear proteins. These findings make Akt/PKB an important therapeutic target for the treatment of cancer, diabetes, laminopathies, stroke and neurodegenerative disease.
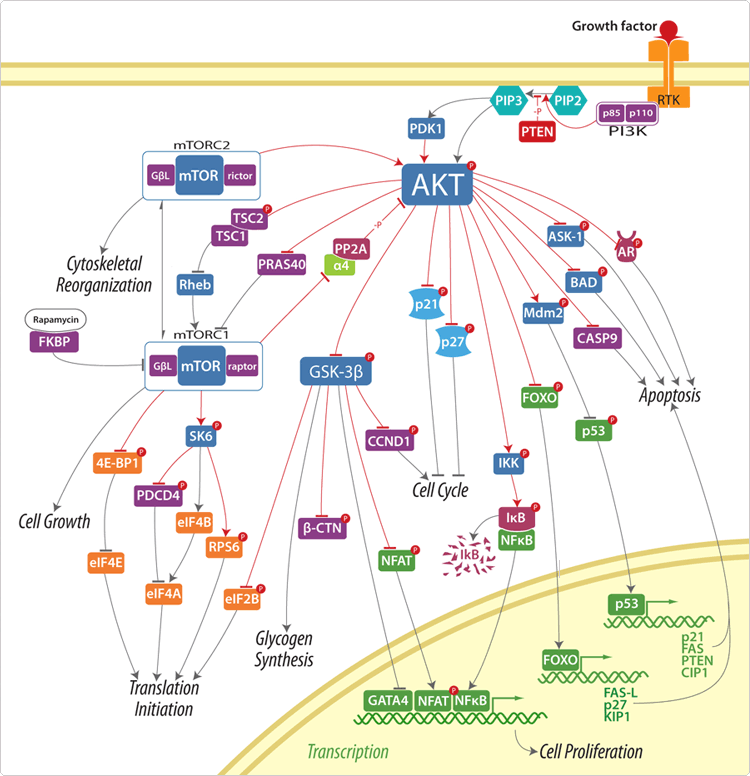
As for the AKT in the pathway, it seems that like the mTOR, it is also another serine/threonine protein kinase. From the Wikipedia article on AKT…
Akt, also known as Protein Kinase B (PKB), is a serine/threonine-specific protein kinase that plays a key role in multiple cellular processes such as glucose metabolism, apoptosis, cell proliferation, transcription and cell migration.
Function
Akt regulates cellular survival and metabolism by binding and regulating many downstream effectors, e.g. Nuclear Factor-κB, Bcl-2 family proteins and murine double minute 2 (MDM2).
Cell survival: Overview of signal transduction pathways involved in apoptosis.
Akt could promote growth factor-mediated cell survival both directly and indirectly. BAD is a pro-apoptotic protein of the Bcl-2 family. Akt could phosphorylate BAD on Ser136,[13] which makes BAD dissociate from the Bcl-2/Bcl-X complex and lose the pro-apoptotic function. Akt could also activate NF-κB via regulating IκB kinase(IKK), thus result in transcription of pro-survival genes.
Cell Cycle: Akt is known to play a role in the cell cycle. Under various circumstances, activation of Akt was shown to overcome cell cycle arrest in G1 and G2 phases. Moreover, activated Akt may enable proliferation and survival of cells that have sustained a potentially mutagenic impact and, therefore, may contribute to acquisition of mutations in other genes.
The PI3K stands for the term “Phosphatidylinositide 3-kinases (PI 3-kinases or PI3Ks)”. From the Wikipedia article on the PI3K…
“….are a family of enzymes involved in cellular functions such as cell growth, proliferation, differentiation, motility, survival and intracellular trafficking, which in turn are involved in cancer. PI3Ks are a family of related intracellular signal transducer enzymes capable of phosphorylating the 3 position hydroxyl group of the inositol ring of phosphatidylinositol (PtdIns). They are also known as phosphatidylinositol-3-kinases. The pathway, with oncogene PIK3CA and tumor suppressor PTEN (gene), is implicated in insensitivity of cancer tumors to insulin and IGF1, in calorie restriction.
Function
PI 3-kinases have been linked to an extraordinarily diverse group of cellular functions, including cell growth, proliferation, differentiation, motility, survival and intracellular trafficking. Many of these functions relate to the ability of class I PI 3-kinases to activate protein kinase B (PKB, aka Akt) as in the PI3K/AKT/mTORpathway. The p110δ and p110γ isoforms regulate different aspects of immune responses. PI 3-kinases are also a key component of the insulin signaling pathway.”
Conclusion:
At this point, the spiderweb on how each protein or enzyme or kinase interact with each other is super complicated. I do understand that the arrows tell you whether the beginning component will either inhibit or promote the second component. From the diagram below here, we can make a very general outline on how the PI3K/AKT/mTOR Signaling pathway works without going into the big details to keep things simple, at least for right now. You have a growth factor (like insulin, IGF-1 or IGF-2) coming in to reach past the membrane that surrounds each cells that holds the cytoplasm inside. It has to pass through the bi-lipid layer. Once it gets past the layer, it will get into the cell and this is where the intracellular pathway will happen. Somehow a p110 and p85 is trigger, which will result in the triggering of something known as PIP2 allong with them to create or promote PIP3. This leads to the AKT kinase being trigger. That triggers the mTOR kinase. Eventually all of the cascading pathway has a result on the way the nucleus DNA does its translating, transcription, gene expression, and protein synthesis. It is clear from the number of interactions, lines, and arrows that each of the major elements in this pathway has multiple (and I mean MULTIPLE) roles and functions in the development, growth, proliferation, and differentiation of cells.

Pingback: Increase Body Regeneration Ability Through Knockout of Genes Controlling Wnt Beta Catenin Pathway | Natural Height Growth