In recent news for the last week, there was reports coming out about the fact that a team of researchers in Oregon (Oregon Health & Science University) managed to be able to produce stem cells from cloning human embryos for the first time.
NBC News: Cloning technique produces human stem cells for the first time
What I wanted to talk about in this post is just how this type of news, about the fascinating new branch of biomedical research, will possibly effect the height increase endeavor for the coming years and even decades.
First, what is the endeavor of even doing stem cells research in the first place, at least for this group of researchers?
Answer: “…use cloning technology to make human embryos and grow stem cells from them in the hopes of making perfectly matched grow-your-own tissue transplants.”
The article writer goes into a very superficial description of what the researchers did with….
“They used a human egg cell and parts of a human skin cell to grow a very early human embryo, then transformed cells from this ball of cells into beating heart cells and skin cells. The process may eventually help treat a range of diseases, from Parkinson’s to rare inherited conditions, they reported Wednesday…”
The scientific abstract they referenced to from the journal Cell was “Human Embryonic Stem Cells Derived by Somatic Cell Nuclear Transfer”
Cell, 15 May 2013
Copyright © 2013 Elsevier Inc. All rights reserved.
10.1016/j.cell.2013.05.006
Authors
Summary
Reprogramming somatic cells into pluripotent embryonic stem cells (ESCs) by somatic cell nuclear transfer (SCNT) has been envisioned as an approach for generating patient-matched nuclear transfer (NT)-ESCs for studies of disease mechanisms and for developing specific therapies. Past attempts to produce human NT-ESCs have failed secondary to early embryonic arrest of SCNT embryos. Here, we identified premature exit from meiosis in human oocytes and suboptimal activation as key factors that are responsible for these outcomes. Optimized SCNT approaches designed to circumvent these limitations allowed derivation of human NT-ESCs. When applied to premium quality human oocytes, NT-ESC lines were derived from as few as two oocytes. NT-ESCs displayed normal diploid karyotypes and inherited their nuclear genome exclusively from parental somatic cells. Gene expression and differentiation profiles in human NT-ESCs were similar to embryo-derived ESCs, suggesting efficient reprogramming of somatic cells to a pluripotent state.
Analysis #1: On a first take at looking at what is written by the journalist aka non-geneticist, they do a reasonable job in explaining the idea of stem cells to the non biologist. It is well known from high school biology that to make the initial human baby, we need two copies of human dna that would be encased in a specific type of cell. For humans, there is the male and female sex cells. The biological name for human sex cells is gametes. The female gamete is the ovum (or egg) and the male gamete is the sperm. It required the sperm which is much smaller than the egg to borrow itself into the female egg and then inject its DNA within the egg’s nucleus to the egg’s DNA to start the initial spark of the human organism’s creation.
The combined result is known as the zygote. from the Wikipedia article on the Zygote…
“…In multicellular organisms, it is the earliest developmental stage of the embryo. In single-celled organisms, the zygote divides to produce offspring, usually through meiosis. Meiosis is the formation of gametes. Mitosis is the process of cell division….”
and
“All mammals go through the zygote stage of life. Mammalian zygotes eventually develop into a blastocyst, after which they are more generally termed an embryo, and then a fetus.
A human zygote exists as a single cell before undergoing cleavage, forming blastomeres,[5] and becomes a blastocyst on the fifth day.”
This shows that for the human, a mammal, the way we can visualize it can be like….
Sperm + Egg (Ovum) = Zygote (undergoing cleavage) —> Blastomeres —> Blastocyst —> Embryo —> Fetus —> Baby (prenatal) —> actual birth —> Baby (neonatal).
What I had found out from doing gene therapy research was that apparently one can do genetic engineering not just on one cell (ie a single zygote) at a time but that genetic engineering can be done on multiple cells at the same time, at least when the multicellular organism is still ni the infancy of its development which would be when it is still making the blastomeres or in the blastocyst stage.
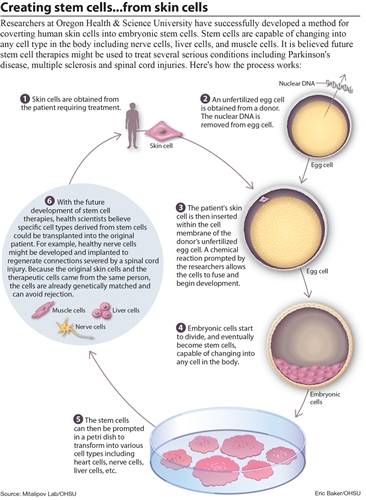
What the University researchers did was to combine the human egg and then one skin cell from explanted adult human skin tissue. The DNA in the skin cell was taken and injected deep into the nucleus of the egg so that the normal fertilization process would start. The very early embryo the author is talking about is probably the blastocyst that develops after a few days from the initial combination.
Note what the article quotes next….
 “These stem cells are kind of very early unprogrammed cells but they have the capacity to become any other cell type,” says Shoukhrat Mitalipov, who led the research.
“These stem cells are kind of very early unprogrammed cells but they have the capacity to become any other cell type,” says Shoukhrat Mitalipov, who led the research.
These cells are very different from so-called adult stem cells, like those taken from bone marrow. Adult stem cells cannot give rise to cells of other tissue types — blood cells cannot be used to make brain cells, for instance.
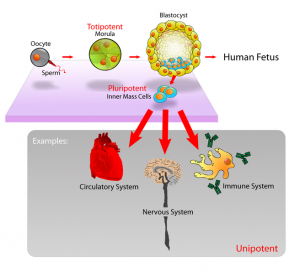
This shows another important point about stem cells. There are different types of stem cells. We have mostly talked about mesenchymal stem cells that can differentiate into the chondrocytes and then the cartilage tissue we want. However the stem cells are still not as powerful as the stem cells one can find in embryos, which are termed pluripotent. pluripotent stem cells can differentiate into any type of cell found in the human body. The stem cells of the human bone marrow have a limited range of cells that they turn into. For our endeavor, maybe we have to find the more powerful types of stem cells needed since maybe the mesenchymal stem cells in our intermedullary cavities are already too old or “differentiated” to far to be effective. The article does quote one of the researchers on the team…
“These stem cells are kind of very early unprogrammed cells but they have the capacity to become any other cell type,” says Shoukhrat Mitalipov, who led the research.”
The reason these guys chose to use very early stage cells was to have more power/ability for the cells.
From looking at the diagram picture above by the people who wrote the article (all credit goes to them) it seems that my initial analysis was wrong about what they did. They seem to extract the nucleus from the skin cell, not the DNA as I said. That actually makes more sense because one can’t extract the entire chromosome set very easily, much less the DNA strands. It makes more sense to remove the nucleus. So the nucleus is removed from the skin cell.
The Ovum/Egg has its own nucleus removed. Maybe there was some very small, very sharp, and accurate pliers or tongs to remove it. After the nucleus in the egg is removed, the nucleus from the skin is implanted. The technique that the researchers used was known as “somatic cell nuclear transfer”. The thing about why this technique seems to succeed where cloning of humans didn’t work before was because the donated human egg provides fresh & rejuvenating DNA.
It seems that groups of researchers have tried before to clone humans or at least make stem cells that they can use to make organs and tissues that can be implanted in humans who suffer debilitating injuries. There have been groups of researchers who have tried to use the “leftover” stem cells in fertility clinics. There have been other researchers who try to “trick” ordinary skin cells into remodelling themselves into different tissues.
I will use a diagram I took from the Wikipedia article on Stem Cell Potency…
What we are seeing is that
Something to note here is the term “Oocyte”. From the Wikipedia article on oocytes…
An oocyte, oöcyte, ovocyte, or rarely ocyte, is a female gametocyte or germ cell involved in reproduction. In other words, it is animmature ovum, or egg cell. An oocyte is produced in the ovary during female gametogenesis. The female germ cells produce a primordial germ cell (PGC) which undergoes mitosis to form an oogonium. During oogenesis the oogonium becomes a primary oocyte.
Analysis #2:
Let’s go back to the scientific article that was cited, Human Embryonic Stem Cells Derived by Somatic Cell Nuclear Transfer
Summary
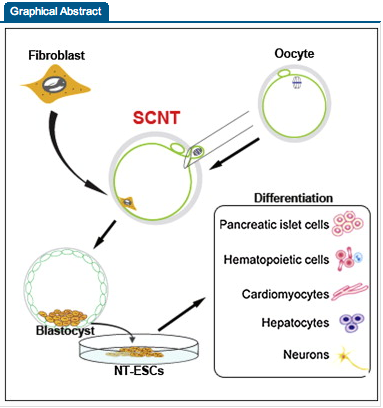 Reprogramming somatic cells into pluripotent embryonic stem cells (ESCs) by somatic cell nuclear transfer (SCNT) has been envisioned as an approach for generating patient-matched nuclear transfer (NT)-ESCs for studies of disease mechanisms and for developing specific therapies. Past attempts to produce human NT-ESCs have failed secondary to early embryonic arrest of SCNT embryos. Here, we identified premature exit from meiosis in human oocytes and suboptimal activation as key factors that are responsible for these outcomes. Optimized SCNT approaches designed to circumvent these limitations allowed derivation of human NT-ESCs. When applied to premium quality human oocytes, NT-ESC lines were derived from as few as two oocytes. NT-ESCs displayed normal diploid karyotypes and inherited their nuclear genome exclusively from parental somatic cells. Gene expression and differentiation profiles in human NT-ESCs were similar to embryo-derived ESCs, suggesting efficient reprogramming of somatic cells to a pluripotent state.
Reprogramming somatic cells into pluripotent embryonic stem cells (ESCs) by somatic cell nuclear transfer (SCNT) has been envisioned as an approach for generating patient-matched nuclear transfer (NT)-ESCs for studies of disease mechanisms and for developing specific therapies. Past attempts to produce human NT-ESCs have failed secondary to early embryonic arrest of SCNT embryos. Here, we identified premature exit from meiosis in human oocytes and suboptimal activation as key factors that are responsible for these outcomes. Optimized SCNT approaches designed to circumvent these limitations allowed derivation of human NT-ESCs. When applied to premium quality human oocytes, NT-ESC lines were derived from as few as two oocytes. NT-ESCs displayed normal diploid karyotypes and inherited their nuclear genome exclusively from parental somatic cells. Gene expression and differentiation profiles in human NT-ESCs were similar to embryo-derived ESCs, suggesting efficient reprogramming of somatic cells to a pluripotent state.
Interpretation
What we see is that this experimental study somehow managed to figure out around the decade old problem on how to create stable human nuclear transfer-embryonic stem cells (NT-ESCs)
As states in the introduction of the article…
In humans, SCNT was envisioned as a means of generating personalized embryonic stem cells from patients’ somatic cells, which could be used to study disease mechanisms and ultimately for cell-based therapies (Lanza et al., 1999; Yang et al., 2007). However, the derivation of human nuclear transfer-embryonic stem cells (NT-ESCs) has not been achieved despite numerous attempts during the past decade. The roadblock responsible for failure is early embryonic arrest of human SCNT embryos precluding derivation of stable NT-ESCs. Typically, SCNT embryos fail to progress beyond the eight-cell stage, presumably due to an inability to activate critical embryonic genes from the somatic donor cell nucleus (Egli et al., 2011;Noggle et al., 2011)
After noticing in lab rhesus monkeys that their fibroblast skin cells could be reprogrammed into the NT-ESCs they were looking for so they state “…Therefore, we reasoned that, similar to other mammals, human MII oocytes must contain reprogramming activity.”
This is where they got the idea of using the very early stage of the ovum right before fertilization and removing the nucleus for its reprogramming activity.
For the researchers who are going to use the stem cells that make tissue for medical reasons , I agree with the general idea that the best option for people is to become their own donors since it seems that it is much harder for some people to find that perfect donor for them.
As stated in the article….
“…using a patient’s own cells offers potentially huge advantages. “A lot of patients don’t have an optimal donor,” he said. So bone marrow transplants are done only for the patients in the most dire need.
“If we could make every patient their own donor … we would bypass the transplant barrier,” he said. “Everyone could be a donor for themselves.””
Implications For Height Increase Research:
We found that from our bone marrow, which is what is inside the long bone intermedullary cavity, some types of stem cells which have limited differentiating ability. They can differentiate into chondrocytes if we really focused on just finding the right growth factors to induce them into the chondrogenic lineage, although it seems that most growth factors are both osteogenic and chondrogenic. If we can get the stem cells ,in say the epiphysis or diaphysis, to turn chondrogenic, there is still not enough time for them to form the cartilage tissue needed to expand and push the bones longitudinally before turning into the osteblasts and osteocytes. The recent news about this group of university researchers in oregon makes me think about the possibility of injecting the newly created stem cells using somatic cell nuclear transfer into human bone for to form cartilage tissue or growing the blastocytes derived into epiphyseal hyaline cartilage. This approach may be the easiest way to make real growth plates in the lab using non-controversial methods like taking the pluripotent stem cells from embryos.