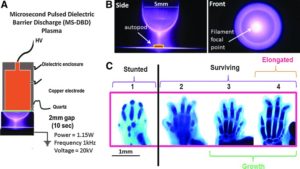
Non-Thermal plasma is plasma that is not in thermodynamic equilibrium. Atmospheric pressure plasma matches the pressure of the surrounding atmosphere.
Non-Thermal Atmospheric Pressure Plasma Enhances Mouse Limb Bud Survival, Growth and Elongation.
“appendage regeneration is dependent on reactive oxygen species (ROS) production and signaling. Mesenchymal cell stimulation by non-thermal (NT)-plasma, which produces and induces ROS, would (1) promote skeletal cell differentiation and (2) limb autopod development. Stimulation with a single treatment of NT-plasma enhanced survival, growth and elongation of mouse limb autopods in an in vitro organ culture system. Noticeable changes included enhanced development of digit length and definition of digit separation. These changes were coordinate with enhanced Wnt signaling in the distal apical epidermal ridge (AER) and presumptive joint regions. Autopod development continued to advance up to 144 hr in culture, seemingly overcoming the negative culture environment normally observed in this in vitro system. Real time qPCR analysis confirmed the up-regulation of chondrogenic transcripts. Mechanistically, NT-plasma increased the number of ROS positive cells in the dorsal epithelium, mesenchyme and the distal tip of each phalange behind the AER, determined using dihydrorhodamine. The importance of ROS production/signaling during development was further demonstrated by the stunting of digital outgrowth when anti-oxidants were applied. NT-plasma initiated and amplified ROS intracellular signaling to enhance development of the autopod. Parallels between development and regeneration, suggest the potential use of NT-plasma could extend to both tissue engineering and clinical applications to enhance fracture healing, trauma repair and bone fusion.”
“Vertebrate limbs grow through the coordinated activity of numerous growth factor signaling pathways, including the Wnt, fibroblast growth factor (FGF), bone morphogenetic protein (BMP), Notch, and transforming growth factor-β pathways. ROS play a primary role in mediating the activity of these factors and/or are integrally involved in regulating downstream signaling pathways. For example, a sustained increase in ROS levels is required for Wnt β-catenin signaling and the activation of one of its main downstream targets, fgf20. In addition, naturally occurring oxidative spikes are observed in most of the transition stages throughout developmental and regenerative processes. Furthermore, there is precedence for ROS production to both direct and enhance mesenchymal cell differentiation into either chondrocyte or osteoblast lineages”
“Since the NT-plasma discharge occurs in air, it is a highly complex mix of reactive chemical species (i.e., nitric oxide [NO], ROS, oxygen singles, and ozone), free ions, visible, ultraviolet, and near-infrared light, electric fields, and electromagnetic (EM) radiation.”
“one study showed that a 2 h treatment with EM field stimulated limb growth through increased proliferation and chondrocyte differentiation”
That is an absolutely huge elongation.
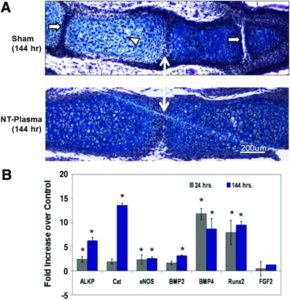
“Morphological formation of the joint region and alcian blue staining of proteoglycan appeared normal in sham and NT-plasma-treated digits. Chondrocytes in the proximal segment of the sham-treated digit were entering the prehypertrophic stage, based on the larger size, greater cellular spacing, and decrease in proteoglycan (white arrowhead). This typically precedes the formation of the primary center of ossification in the diaphysis. In contrast, chondrocytes from the NT-plasma-treated digit were immature and contained large amounts of proteoglycan. Even more striking was the much larger cartilage phalangeal segments in the NT-plasma-treated digit. A white line with double arrows marks the joint between the first and second phalanges in both sections. In the sham-treated digit, the adjacent joint spaces (white arrow) can be clearly observed; whereas in the NT-plasma-treated digits, there is no evidence of the next joint space, due to the increased segment length.”<-So maybe the plasma treatment increases stem cell differentiation to chondrocytes which is what we’d be looking for.
“Histology and quantitative real time-polymerase chain reaction were used to assess chondrogenesis and molecular events associated with the accelerated development. (A) A representative, hematoxylin and eosin (H&E) and alcian blue stained sections of middle phalanges from sham and NT-plasma-treated limb autopods. A white double arrow line marks the first joint space. The white arrows mark adjacent joint spaces, and the white arrowhead marks prehypertrophic chondrocytes in the sham control. (B) At 24 h post-NT-plasma treatment, alkaline phosphate (ALKP), catalase (CAT), endothelial nitric oxide synthase (eNOS), and bone morphogenetic protein 2 (BMP2) expression increased twofold above control. BMP4 and runt-related transcription factor 2 (Runx2) increased 12-fold and 8-fold, respectively, and fibroblast growth factor (FGF)-2 showed no increase. One hundred forty-four hours later, increases for ALKP (6-fold), CAT (14-fold), and BMP2 (3.5-fold). eNOS remained increased twofold above control at 144 h. BMP4 and Runx2 showed a relatively consistent elevated expression at 24 and 144 h. The results are expressed as the mean±standard deviation (n=3 [two limbs pooled per sample], *p<0.05). ”
“NT-plasma treatment promoted both growth and differentiation of cartilaginous elements within the embryonic mouse autopod. Based on the lack of limb growth after NT-plasma treatment in an antioxidant environment, and the increase in H2O2-positive cells in and under the epithelia throughout the 6 day culture period, we propose that NT-plasma induction of intracellular ROS also plays a role in the growth and differentiation observed in the autopod. In addition, H2O2-positive cells were observed in the distal digit tips behind the AER, concurrent with enhanced Wnt signaling. These findings are consistent with the ROS dependence of signaling factors (e.g., Wnt, BMP, and FGF) required for autopod development.”
“ROS production may not be the sole mechanism driving enhanced autopod growth. A second possible reason that NT-plasma enhanced autopod growth was its inhibition of epithelial overgrowth. ”
“In limb regeneration, a thickening of the epidermis has been shown to inhibit signaling to the blastema, halting the regeneration process.”
Hard to determine causality as there are so many potential causal factors as there are so many confounding variables in plasma.