Is some of the recent scientific advancements hiding key features that may give the secrets to growth? Compare this recent study to a 1920s study that provides an insight about adult longitudinal bone growth related to bone softening.
Development of a protocol to quantify local bone adaptation over space and time: Quantification of reproducibility.
“In vivo micro-computed tomography (µCT) scanning of small rodents is a powerful method for longitudinal monitoring of bone adaptation{May be helpful to measure LSJL?}. However, the life-time bone growth in small rodents makes it a challenge to quantify local bone adaptation. Therefore, the aim of this study was to develop a protocol, which can take into account large bone growth, to quantify local bone adaptations over space and time. The entire right tibiae of eight 14-week-old C57BL/6J female mice were consecutively scanned four times in an in vivo µCT scanner using a nominal isotropic image voxel size of 10.4µm. The repeated scan image datasets were aligned to the corresponding baseline (first) scan image dataset using rigid registration. 80% of tibia length (starting from the endpoint of the proximal growth plate) was selected as the volume of interest and partitioned into 40 regions along the tibial long axis (10 divisions) and in the cross-section (4 sectors). The bone mineral content (BMC) was used to quantify bone adaptation and was calculated in each region. All local BMCs have precision errors (PE%CV) of less than 3.5% (24 out of 40 regions have PE%CV of less than 2%), least significant changes (LSCs) of less than 3.8%, and 38 out of 40 regions have intraclass correlation coefficients (ICCs) of over 0.8. The proposed protocol allows to quantify local bone adaptations over an entire tibia in longitudinal studies, with a high reproducibility, an essential requirement to reduce the number of animals to achieve the necessary statistical power. ”
“in rodents like mouse, bone growth spans across the animal׳s life time”
Here’s a 1926 study regarding the regeneration of bone:
THE REGENERATION OF BONE
An early theory of the bone: “the “juice” of bone is released by a fracture,
fills the gap between the ends and there solidifies.”
“The excision of a necrotic bone (the modern sequestrectomy) was more than once reported with complete reconstitution of the affected bone.”
“bone regeneration depends upon the periosteum. the thickening of this membrane and its later consolidation into bone in experimental fractures and drill holes.”
The four main elements of adult bone: bone cells; periosteal cells; endosteal cells; calcified matrix.
The marrow cavity of a bone(that which contains the juice) is broken up by the trabeculae of spongy bone.
“the bony shell or cortex, we find a definite architecture. On the inner and outer surfaces, the matrix appears in layers lying parallel to the surface. Further in, these layers lose the parallel arrangement and are there grouped in a series of concentric circles. In the center of each of these circles is an opening, the haversian canal, containing a blood vessel and lined with a membrane. Throughout the substance of the matrix between these canals and in the
parallel layers are numerous smaller openings, the lacunae, in which lie the bone cells.”
“Enveloping the outer aspect of the bone is the periosteum, a membrane consisting
of several layers of cells resembling connective tissue cells, with an enlongated nucleus and abundant fibrillar intercellular substance.”
“solid bone will not grow in length from within by expansion. The calcified matrix cannot
expand. Whatever growth takes place must take place by accretion.”
“Completely calcified bone will not grow interstitially but soft bone will grow interstitially. The experiment just described, if done on a young bone not yet hard, will result in separation of the nails. This is an observation which has a curious reference to adult pathology. Bones in certain pathologic states that render them soft, will occasionally increase in length long after the epiphyseal cartilages are united. This growth is undoubtedly a genuine interstitial addition of new bone, a true expansion of the matrix exactly comparable to such growth in young bones. The commonest conditions in which this phenomenon is encountered are osteomyelitis, Paget’s distlase of bone (osteitis deformans) and Recklinghausen’s disease (neurofibromatosis).
all of which result in a certain degree of decalcification, that is a softening of the bone”
“bones assume a shape adapted to the function they are to perform.”
“Suppose a small drill passed through the cortex of a bone into the marrow cavity. The first effect, of course, is hemorrhage. On withdrawing the drill, the defect fills with blood. Within a few hours this clot begins to be replaced by a plug of fibrin containing polymorphonuclear
leucocytes. Fibrin is the culture medium, so to speak, of young tissue. Then appears a proliferation of the periosteal and endosteal cells, the production of periosteal and endosteal fibroblasts which differ from connective tissue fibroblasts only in their inherent capability of producing bone. These proliferating cells heap up at the edges of the defect, producing a macroscopic thickening of the periosteum and endosteum, and then grow down and up respectivcly into the fibrin that fills the defect, until it is completely replaced. The
periosteal and endosteal reaction extends on the surfaces of the bone over a considerable distance from the point of injury.”
“Before the process of fibrosis is complete, ossification begins at the points where the periosteal and endosteal proliferation began, namely over the surfaces of the bone at the edges of the defect.”
“when a foreign body is brought into contact with bone, one must anticipate that the regenerative processes will be diminished and retarded and that actual bone destruction may occur.”
A discussion attached to the end of the paper suggests that periosteums function merely is a membrane that constrains the bone and that it’s the fluid within the bone that results in bone regeneration. The periosteum also serves as a mechanism of communication with other tissues. The commenter stated that the periosteum only appears after the bone has taken place.
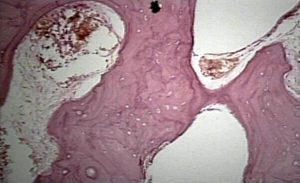
Here’s the bone histology for Paget’s disease:
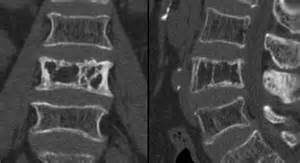
Here’s Paget’s disease in an x-ray of the spine:
Here’s someone with paget’s disease in the arm:
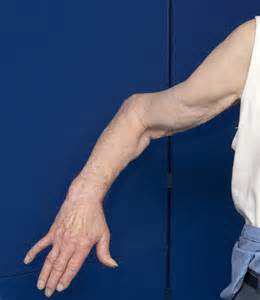
Paget’s disease can make bones longer but not in a real symmetrical way so it’s not really appealing.
Paget’s disease is a disease where bone undergoes break down and rebuilding much faster than normal(so basically faster bone remodeling cycle) and this results in weaker bones as bone does not have time to develop strength. If there was a way that Paget’s disease was found to increase height we could mimic the effects somewhat by increasing the amount of bone remodeling that occurs.
Osteomylitis seems caused by infection which would be hard to manage.
Neurofibromatosis can cause overgrowth of specific extremeties related to NF1 and NF2 mutations.