New LSJL study was published that has some good stuff. Maybe the paper will finally give the insight on how to use mechanical loading to gain height.
Loading-driven PI3K/Akt signaling and erythropoiesis enhanced angiogenesis and osteogenesis in a postmenopausal osteoporosis mouse model
“Bone vasculature influences osteogenesis and haematopoiesis in the bone microenviroment. Mechanical loading has been shown to stimulate the formation of osteogenesis-related type H vessels in an ovariectomy (OVX)- induced osteoporosis mouse model. To determine the loading-driven mechanism of angiogenesis and the formation of type H vessels in bone, we evaluated the roles of PI3K/Akt signaling and erythropoiesis in the bone marrow. The daily application of mechanical loading (1 N at 5 Hz for 6 min/day) for 2 weeks on OVX mice inhibited osteoclast activity{although osteoclast activity is good for our purposes as degradation of bone is needed to theoretically create a new growth plate}, associated with an increase in the number of osteoblasts and trabecular volume ratio. Mechanical loading enhanced bone vasculature and vessel formation, as well as PI3K/Akt phosphorylation and erythropoiesis in the bone marrow. Notably, LY294002, an inhibitor of PI3K signaling, blocked the tube formation by endothelial progenitor cells, as well as their migration and wound healing. The conditioned medium, derived from erythroblasts, also promoted the function of HUVECs with elevated levels of VEGF, CD31, and Emcn. Collectively, this study demonstrates that mechanical loading prevents osteoporotic bone loss by promoting angiogenesis and type H vessel formation. This load-driven preventing effect is in part mediated by PI3K/Akt signaling and erythropoiesis in the bone marrow”
“A specific vessel subtype called a type H vessel, which is positive for CD31 and endomucin (CD31hiEmcnhi), has been reported to link angiogenesis with osteogenesis”
“Vascular-forming endothelial cells provide a framework of bone homeostasis and metabolism, acting as a cellular highway for blood cells, leukocytes, and other types of cells throughout the body ”
“Erythropoiesis is characterized by the movement of lineage-committed cells with progenitors, precursors, and mature red blood cell (RBC) compartments, which are located in the fetal liver and the postnatal bone marrow”
“Molecular signals by mechanosensing and transduction, leading to a series of cellular responses. The phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway has been shown to play a critical role in the regulation of diverse cellular functions and is also involved in angiogenesis and erythropoiesis. Our previous study predicted that joint loading increases the expression of Akt, which may promote bone formation and homeostasis. The effect of PI3K signaling on endothelial progenitor cells (EPCs) in the bone marrow of OVX mice, however, has not yet been demonstrated in response to joint loading. ”
“we hypothesized that the reduction in type H vessels and the hematopoietic function in OVX mice is suppressed by mechanical loading through PI3K/Akt signaling, which stimulates erythroid differentiation of hematopoietic stem cells (HSC) and promotes vessel formation”<-so joint loading may not suppress osteoclast activity in normal bone as the OVX environment is different.
“Ninety female C57BL/6 mice (~16 weeks of age, Animal Center of Academy of Military Medical Sciences, China) were used. Mice were randomly divided into three groups: the sham control group (Sham, n = 30), the ovariectomized group (OVX, n = 30), and the loading-treated ovariectomized group (OVXL, n = 30).”<-unfortunately no loaded control group.
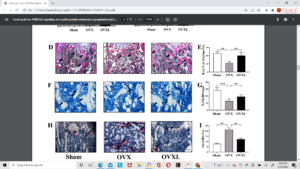
Above is histological slides. Can’t really see anything abnormal. There may be something atypical going on in F where the added arrow is pointing.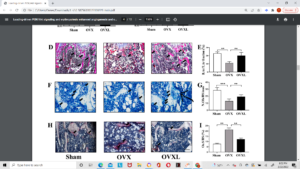
According to figure 2G PI3K, AKT, and VEGF activity was greatly enhanced by joint loading even over controls. But according to 2H, I, J it did not increase the ratios of phosphorylated PI3K and AKT to total PI3K and AKT and VEGF relative to Beta-Actin.
“The formation of type H vessels was correlated to the stimulation of osteogenesis, and the result suggests that the promotion of type H formation and angiogenesis are important in response to load-driven suppression of OVX-induced bone loss. ”
“knee loading stimulated PI3K/Akt phosphorylation in the bone, and this stimulation can be linked to loading-driven bone metabolism”<-although this alone is definitely not sufficient to induce new longitudinal bone growth.
“Knee loading enhanced cell migration and wound healing”
“in OVX mice, load-driven angiogenesis via erythropoiesis contributed to suppressing the loss of osteoporotic bone. “<-erythropoiesis is the creation of new red blood cells. Erythropoiesis is thought to not typically occur in adult bones so if joint loading can induce new erythropoiesis that is a big deal.
The mice here were subjected to 1n loading at 5Hz for 6 min/day. This is actually higher than that performed on the original bone lengthening LSJL study 5-min bouts at 0.5 N[at 5Hz).
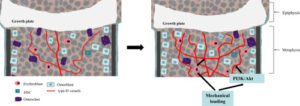
Here’s a graphical representation in the study at how mechanical loading might stimulate bone:
The estimated increase in type H vessels is dramatic. Osteoclast activity as depicted is way done too.
And here’s the description of type H vessels “Type H vessels are noted most abundantly in young, growing animals, adjacent to the growth plate. The elongation of long bones by the process of endochondral ossification occurs at the growth plate and involves continuous replacement of cartilage matrix by calcified bone to acquire their typical shapes through bone modeling.”
Could type H vessels be the key for new longitudinal bone growth here’s a study explaining type H vessels.
Type H blood vessels in bone modeling and remodeling
“In the mammalian skeletal system, osteogenesis and angiogenesis are intimately linked during bone growth and regeneration in bone modeling and during bone homeostasis in bone remodeling. Recent studies have expanded our knowledge about the molecular and cellular mechanisms responsible for coupling angiogenesis and bone formation. Type H vessels, termed such because of high expression of Endomucin (Emcn) and CD31, have recently been identified and have the ability to induce bone formation. Factors including platelet-derived growth factor type BB (PDGF-BB), slit guidance ligand 3 (SLIT3), hypoxia-inducible factor 1-alpha (HIF-1α), Notch, and vascular endothelial growth factor (VEGF) are involved in the coupling of angiogenesis and osteogenesis.”
“in bone modeling associated with endochondral ossification, hypertrophic chondrocytes express high levels of vascular endothelial growth factor (VEGF), which promotes the vascular invasion of cartilage, recruits chondroclasts to resorb hypertrophic cartilage and osteoblasts to build the bone matrix”
“In bone modeling in response to mechanical loading, macrophage/non-resorbing osteoclast lineage cells secrete platelet-derived growth factor type BB (PDGF-BB) to recruit both endothelial and osteoblast precursor cells to couple angiogenesis with osteogenesis”
“activation of mechanistic target of rapamycin complex (mTORC) in articular chondrocytes promoted VEGF secretion into subchondral bone and stimulated type H vessel formation.”
“type H vessels were reported to digest the cartilage matrix, which may be another mechanism of cartilage degeneration in OA.”
“In fracture healing, type H vessels appear during the stage of fibrocartilaginous callus formation. Type H vessels form in the fracture callus and induce bone formation. Factors, such as SLIT3, can promote fracture healing by augmentation of type H vessel induced osteogenesis.”<-Limb lengthening basically if LSJL can induce type H vessels and if type H vessels are important in neocartilage formation than that would be a big find. But I can only find that Type H vessels are important in absorbing the cartilage matrix not in creating the cartilage matrix. Although increased Type H Vessels as shown in this study could be indicative of enhanced and maybe even new growth plate activity would would be massive. IF the mice got new Type H blood vessels past skeletal maturity that would be a huge find 16 week of the type used in this study are pretty far along skeletal wise so new type H vessels is still a big deal but they are still not quite old enough.