Here are David Burr’s thoughts on the study. I have spoken to David Burr before as he’s add that microdamage in bone does not heal by endochondral ossification. He’s said: “Diffuse damage(and linear microcracks that are are not fully fractures) don’t heal by endochondral ossification.” “Large cracks always heal by remodeling, but full fractures, trabecular or otherwise, can heal by endochondral ossification.” I pointed out this study to him given that he’s made the above statements. Here’s what he had to say: “The endochondral ossification and chondrocytes that are present here are in woven bone. That is not unusual. There is none of that in the pre-existing bone. Several things here are remarkable (though not that cartilage can appear in woven bone). One question is why is there woven bone when the strains are only 640 microstrain (not high, as suggested by the authors). This suggests there was some independent effect of muscle stimulation on bone, beyond the level of strain. This could be due to the manner of stimulation, or to the fact that these were quite young rats – not adult – started at 8 weeks, or that the muscle stimulation occurred consistently over a long period of time. At some point in the very distant past, we showed that high strains (which were not present here) in dogs can cause woven bone formation without fracture. It is simply a response either to a need to adapt quickly, or to damage that is not evidenced in a fracture. So, I don’t find anything particularly novel about this, and some fundamental flaws in the model and analysis.”<-I think Burr is arguing that endochondral ossification is only occurring in immature bone that is not under repair.
I suggested that the independent effect of muscle stimulation could be fluid flow. Here’s what he had to say: “Strain is still too low – but it depends on the strain rate (ie, strain x frequency). But, if the frequency is too high, then fluid can’t relax and so the effect is blunted”
I talked to Dan Huey in the past about the possibility of endochondral ossification within the bone.
“”While MSCs derived from bone marrow have shown the ability to differentiate down the chondrocytic lineage both in vitro and in vivo the efficiency and completeness of this process hinders the formation of stable hyaline tissue. Ectopic differentiation of MSCs into chondrocytes does not occur in the marrow cavity due to a lack of the appropriate signals (both mechanical and biochemical). These MSCs are tuned by their environment to contribute to the natural bone remodeling process. However, even when these cells are introduced into a cartilage defect via microfracture, complete chondrocyte differentiation does not occur, as evidenced by the formation of fibrous tissue. For these cell to undergo complete chondrogenesis the proper combination of mechanical and biochemical cues must be provided. As the clot formed in microfracture is quite soft the cells within the clot will not receive the appropriate level of mechanical forces for chondrogenesis. With regards to the biochemical signals, a cartilage stimulating growth factor analagous to BMP’s effect for osteogenic differenetiation has not been identified. With respect to the term microfracture, in cartilage and bone it means two different things. For cartilage microfracture is a surgical procedure that involves creating holes in the bone underlying a cartilage defect to allow stem cells to enact a healing response. With regards to bone, microfractures are the very small breaks in bone that occur during strenuous activity. does not occur in cartilage as it does in bone. In bone, microfracture occurs during strenuous activity and heals.”<-it’s possible that muscular contraction along with other mechanical stimuli could help induce the signals needed for chondrogenic differentiation.
The below study shows the presence of endochondral ossification in non-growth plate regions within bone itself. The problem is that at least according to this stimulus it is not in a longitudinal direction. But this is a huge breakthrough study as it shows that chondrogenic regions can occur outside of the growth albeit in developing rats.
“Muscular contraction plays a pivotal role in the mechanical environment of bone, but controlled muscular contractions are rarely used to study the response of bone to mechanical stimuli. Here, we use implantable stimulators to elicit programmed contractions of the rat tibialis anterior (TA) muscle. Miniature stimulators were implanted in Wistar rats (n = 9) to induce contraction of the left TA every 30 s for 28 days. The right limb was used as a contralateral control. Hindlimbs were imaged using microCT. Image data were used for bone measurements, and to construct a finite-element (FE) model simulation of TA forces propagating through the bone. This simulation was used to target subsequent bone histology and measurement of micromechanical properties to areas of high strain. FE mapping of simulated strains revealed peak values in the anterodistal region of the tibia (640 µε ± 30.4 µε). This region showed significant increases in cross-sectional area (28.61%, p < 0.05) and bone volume (30.29%, p < 0.05) in the stimulated limb. Histology revealed a large region of new bone, containing clusters of chondrocytes, indicative of endochondral ossification. The new bone region had a lower elastic modulus (8.8 ± 2.2 GPa) when compared with established bone (20 ± 1.4 GPa). Our study provides compelling new evidence of the interplay between muscle and bone.”
“eight-week-old Wistar rats (weights 228–282 g) underwent surgical implantation of miniature neuromuscular stimulators”
“Stimulators delivered 0.2 ms pulses at 100 Hz for 200 ms every 30 s, resulting in a total of 9.6 min of stimulation per day. Each 200 ms burst of nerve stimulation at 100 Hz caused a very brief but fused (tetanic, near maximum force) contraction.”<-it would be very hard to induce a maximum force contraction. But muscle stimulation increase fluid forces in bone. So it is not only possible for muscle contraction to stimulate new endochondral ossification within bone but any method that stimulates fluid forces.
“The volume of the stimulated muscles, TA and EDL, showed a significant decrease of volume of, on average, 19% and 16% (p < 0.05), respectively, when compared with the contralateral control limb”<-It’s interesting that the muscle actually decreased in size.
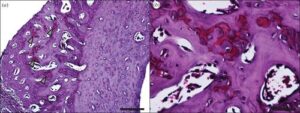
“Histological sectioning targeted to this distal region revealed that the cross-sectional geometry of the contralateral control and the stimulated tibias were markedly different (figure 8a). The stimulated bone shows a large region of primary osteon formation (figure 9). Safranin O staining revealed the presence of clusters of chondrocytes within the region of primary osteon formation!!!!!! (figure 10).”