LSJL does upregulate MATN3. A rosette is a hexameric disc shaped aggregate.
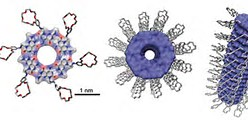
Here’s what a rosette Nanotube looks like. The novel aspect of this is that it can be injected as a liquid.
GROWTH PLATE CARTILAGE REPAIR VIA NOVEL MATRILIN3/ROSETTE NANOTUBE HYBRID MATRIX
“Approximately 15% to 30% of all childhood fractures are growth plate fractures. Because the growth plate determines the length and shape of a mature bone, this type of fracture may result in severe growth abnormalities in children. Pathologically, the growth abnormality is caused by the formation of a bony bridge in the injured growth plate cartilage. Currently, the clinical treatment of growth plate fractures includes the surgical removal of the bony bridge and insertion of autologous fat or cartilage tissue into the empty space to discourage bony bridge reformation. Such surgical procedures are invasive and result in unsatisfactory outcomes. In addition, this treatment is only useful after the bony bridge has formed. Our long-term goal is to understand how to prevent bony bridge formation and improve growth plate cartilage regeneration at cellular and molecular levels and develop the first preventive and therapeutic approach for growth plate fracture. Specifically, the primary objective of this proposal is to evaluate the therapeutic effects of a nano-matrix assembled from matrilin-3 (MATN3) and rosette nanotube (RNT) in a preclinical growth plate fracture model. Our central hypothesis is that the MATN3/RNT nano-matrix specifically promotes chondrocyte growth and enhances chondrogenesis of mesenchymal stem cells (MSCs), while it also inhibits vascularization and osteogenesis at the fracture site{these two things may increase growth plate generation especially since this is supposed to be used for growth plate fracture}. This is the cellular basis for such nano-matrix to improve growth plate cartilage regeneration and prevent bony bridge formation. We will test our central hypothesis and achieve the objective of the proposal by pursuing two specific aims: 1) to determine the ability of MATN3/RNT to prevent bony bridge formation; and 2) to determine the ability of MATN3/RNT to deliver growth factors for further improvement of chondrogenesis and growth plate cartilage regeneration. To achieve the two aims, our overall research strategy includes: 1) optimization of the ratio and dose of MATN3/RNT and its ability and bioactivity for loading growth factors in vitro; and 2) determination of the therapeutic efficay of the nano-matrix in our established growth plate fracture model in rats in long term. The proposed research is innovative: 1) biologically, it simultaneously promotes cartilage regeneration and inhibits bony bridge formation; 2) therapeutically, MATN3 and RNT can be injected as a liquid in a minimally invasive manner, and form a nano- matrix at the fracture site; 3) structurally, the nano-matrix concentrates bioactive MATN3 locally at the fracture site as well as binds TGF-β1 and IGF-1 to achieve multi-functional delivery. With the results of the two specific aims, we expect to 1) realize a synergistic strategy to specifically promote chondrogenesis while inhibiting osteogenesis and vascularization; and 2) develop an injectable approach for the localized delivery of cartilage growth factors. These outcomes have an important positive impact in developing novel, perhaps the first, preventive and therapeutic approach for growth plate cartilage repair. ”
Here’s more info about nanotubes:
Helical rosette nanotubes: a more effective orthopaedic implant material
“Due to the nanometric properties of some physiological components of bone, nanomaterials have been proposed as the next generation of improved orthopaedic implant materials. Yet current efforts in the design of orthopaedic materials such as titanium (Ti) are not aimed at tailoring their nanoscale features, which is now believed to be one reason why Ti sometimes fails clinically as a bone implant material. Much effort is thus being dedicated to developing improved bioactive nanometric surfaces and nanomaterials for biospecificity. Helical rosette nanotubes (HRN) are a new class of self-assembled organic nanotubes possessing biologically-inspired nanoscale dimensions. Because of their chemical and structural similarity with naturally-occurring nanostructured constituent components in bone such as collagen and hydroxyapatite, we anticipated that an HRN-coated surface may simulate an environment that bone cells are accustomed to interacting with. The objective of the present in vitro study is therefore to determine the efficacy of HRN as a bone prosthetic material. Results of this study clearly show that both HRN-K1 and HRN-Arg coated Ti displayed enhanced cell adhesion when compared to uncoated Ti. Enhanced cell adhesion was observed even at concentrations as low as 0.005 mg ml−1. These results point towards new possibilities in bone tissue engineering as they serve as a starting point for further mechanistic studies as well as future manipulation of the outer chemistries of HRN to improve the results beyond those presented here. One such effort is the incorporation of peptide sequences on the outer surface of HRN and/or growth factors known to enhance bone functions. “