Update: I’m still hard at work for a methodology to grow taller. It’s just mostly a lot of self experimentation. Most scientific studies seem to be a lot of more of the same stuff like IGF2 is key.
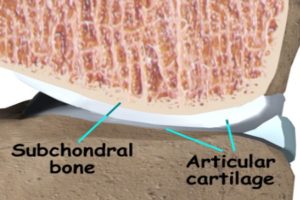
The question is: Can excessive subchondral bone formation make you taller?
Judging from this picture here yes it should.
“Transforming growth factor-β (TGF-β) has been demonstrated as a potential therapeutic target in osteoarthritis. However, beneficial effects of TGF-β supplement and inhibition have both been reported, suggesting characterization of the spatiotemporal distribution of TGF-β during the whole time course of osteoarthritis is important. To investigate the activity of TGF-β in osteoarthritis progression, we collected knee joints from Dunkin-Hartley (DH) guinea pigs at 3, 6, 9, and 12-month old (n = 8), which develop spontaneous osteoarthritis in a manner extraordinarily similar to humans. Via histology and micro-computed tomography (CT) analysis, we found that the joints exhibited gradual cartilage degeneration, subchondral plate sclerosis[a thickening of the subchondral bone where it begins to develop cysts, hardens, and thickens], and elevated bone remodeling during aging. The degenerating cartilage showed a progressive switch of the expression of phosphorylated Smad2/3 to Smad1/5/8, suggesting dual roles of TGF-β/Smad signaling during chondrocyte terminal differentiation in osteoarthritis progression. In subchondral bone, we found that the locations and age-related changes of osterix(+) osteoprogenitors were in parallel with active TGF-β, which implied the excessive osteogenesis may link to the activity of TGF-β. Our study, therefore, suggests an association of cartilage degeneration and excessive bone remodeling with altered TGF-β signaling in osteoarthritis progression of DH guinea pigs.”
“knee osteoarthritis is a disease of the entire joint, including synovitis, meniscal degeneration and malposition, periarticular bone overgrowth{periarticular bone is bone that surrounds the joint overgrowth of this bone should be good for height growth}, and articular cartilage destruction”
” In response to the altered mechanical environment, the bone–cartilage functional unit adjusts the architecture via cells’ adaptations. However, a discrepancy of repair capacity between the chondrocytes and other skeletal cells is thought to further accelerate the progression of osteoarthritis. Furthermore, it is widely appreciated that the subchondral plate and trabecular bone show different responses, where thickening of the plate can be identified along with osteopenic trabeculae at the advanced stage of osteoarthritis”
“ALK5/Smad2/3 route restrains terminal differentiation of chondrocytes, whereas the ALK1/Smad1/5/8 route induces the differentiation. The increase in ALK1/ALK5 ratio in chondrocytes may contribute to the cartilage degeneration.On the other hand, TGF‐β acts as a coupling factor to induce the migration of mesenchymal stem cells (MSCs) to bone resorption sites, implying its potential function in rebalancing bone resorption and formation. Inhibition of TGF‐β signaling in subchondral bone resulted in higher bone quality and less cartilage degeneration in an induced‐osteoarthritis model”
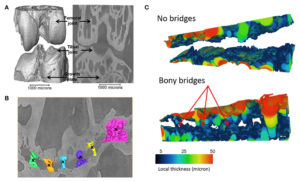
If you look at figure 3 you can see increased subchondral bone height although it should be noted that these pigs were still growing.
“The tidemark advancement is a result of the pathological endochondral ossification at the calcified zone of cartilage”
“Smad2/3 signaling is essential to repress the hypertrophy of chondrocyte, whereas Smad1/5/8 route, namely bone morphogenetic protein (BMP) pathway, is required for chondrocyte terminal differentiation. Inhibition of the Smad1/5/8 signaling pathway led to reduced or delayed chondrocyte hypertrophy. Increase in ALK1/ALK5 ratio was associated with age and osteoarthritis and dominant ALK1/Smad1/5/8 pathway was found in advanced stage of induced osteoarthritis”