A key theory behind LSJL is the use of hydrostatic pressure to induces mesenchymal stem cells into chondrocytes to form new growth plates. Hydrostatic Pressure alters the expression of some of the genes altered by LSJL. Although, there are many genes not shared which is not surprising considering that LSJL exerts other forces than hydrostatic pressure and the microenvironment is different between the rat bone and the cell lines used. Hydrostatic pressure is one of the most consistent stimuli in inducing neo chondroinduction.
Hydrostatic pressure decreases membrane fluidity and lipid desaturase expression in chondrocyte progenitor cells.
“Membrane biomechanical properties are critical in modulating nutrient and metabolite exchange as well as signal transduction. Biological membranes are predominantly composed of lipids, cholesterol and proteins, and their fluidity is tightly regulated by cholesterol and lipid desaturases. To determine whether such membrane fluidity regulation occurred in mammalian cells under pressure, we investigated the effects of pressure on membrane lipid order of mouse chondrogenic ATDC5 cells and desaturase gene expression. Hydrostatic pressure linearly increased membrane lipid packing and simultaneously repressed lipid desaturase gene expression. We also showed that cholesterol mimicked and cholesterol depletion reversed those effects, suggesting that desaturase gene expression was controlled by the membrane physical state itself. This study demonstrates a new effect of hydrostatic pressure on mammalian cells and may help to identify the molecular mechanisms involved in hydrostatic pressure sensing in chondrocytes. ”
“Hydrostatic pressure (HP) is known to reduce lipid membrane fluidity. High HP triggers an adaptive mechanism, during which membrane fluidity is increased by raising the proportion of unsaturated fatty acids.”
“TDC5 cells responded to the change in their membrane fluidity under pressure by modulating the expression of Fads1, Fads2, Scd1 or Scd2. 10 or 20 MPa did indeed significantly inhibit the expression of all four genes after 24 h, while 5 MPa also significantly decreased Fads1 and Scd1 expression.”<-None of the genes were directly altered over significance by LSJL. The genes were measured one hour after loading so it’s possible that the genes returned to baseline after one hour.
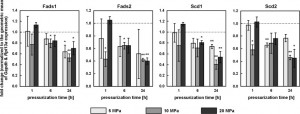 With this data it’s easy to see why there was no change in gene expression level by LSJL as most of the repression of these genes occurred after 6 or 24 hours under hydrostatic pressure.
With this data it’s easy to see why there was no change in gene expression level by LSJL as most of the repression of these genes occurred after 6 or 24 hours under hydrostatic pressure.
“MβCD increased ATDC5 membrane fluidity (laurdan GP values respectively increased and decreased under cholesterol and MβCD treatment”
“Similar to HP, cholesterol increased laurdan GP and significantly inhibited Fads1 and Fads2 expression. By contrast, MβCD[methyl-β-cyclodextrin], which decreased laurdan GP, significantly increased the expression of all four desaturase genes. Together, this suggests that membrane fluidity itself may be the general modulator of desaturase gene expression.”<-Cholesterol has been implicated in controlling endochondral bone growth before.
“the beneficial effects of loading on cartilage are mediated by the transcription factor CITED2, which represses cartilage degradation by the matrix metalloproteinase MMP1.”
“In the human hip joint, loads typically reach 10 MPa during normal activity, with peaks of up to 18 MPa. Considering that interstitial fluid pressure supports about 70 to 90% of the applied load, HP within the cartilage can be expected to regularly exceed 7 MPa and peak at around 16 MPa. 20 MPa is therefore a relatively high pressure”
“How HP is actually sensed by the cell remains poorly understood, but the link between HP and cholesterol suggests at least two hypotheses: both HP and cholesterol may converge on the SREBP pathway, cholesterol by affecting SREBP maturation via SCAP, HP by affecting the membrane fluidity of the endoplasmic reticulum, where non-activated SREBPs reside and which is more sensitive to HP than the plasma membrane; the mechanosensing mechanism of HP could also reside in cholesterol-rich domains like lipid rafts or caveolae”
“membranes rich in unsaturated fatty acids may be compressible enough to deform significantly, and trigger signaling events, even under relatively small pressures. Finally, the plasma membrane is supported by the cytoskeleton, which is mostly incompressible; it is possible that pressurization of the compressible intracellular fluid leads to large deformations in membrane domains unsupported by the cytoskeleton, the change in bulk volume being focused onto a small membrane area.”
