When I wrote ideas on how to possibly create or implant replacement epiphyseal plates for adult height increase, I had talked extensively about the idea on possibly using the articular cartilage layer or the cortical bone proliferation cell layer underneath the periosteum as a initial starting area to regenerate some form of columnar structured growth plate again.
What we see from these next articles is the first example of how it may be possible to regenerate new cartilage from the periosteum itself.
From PubMed, a study entitled “In vivo generation of cartilage from periosteum“… (There is no full text file unless you pay for it)
Tissue Eng. 2005 Mar-Apr;11(3-4):369-77.
In vivo generation of cartilage from periosteum.
Source
Department of Orthopedic Surgery, University Hospital Maastricht, The Netherlands. pj.emans@orthop.unimaas.nl
Abstract
Periosteum has chondrogenic and osteogenic potential and plays an important role in fracture healing. The purpose of this study was to evaluate the reactive tissue formed after damaging the periosteum. Damaging the periosteum may be a way to generate ectopic cartilage or bone, which may be useful for the repair of articular cartilage and bone defects. Periosteum was bilaterally dissected from the proximal medial tibia of New Zealand White rabbits. Reactive periosteal tissue was harvested 10, 20, and 40 days postsurgery and analyzed for expression of collagen types I, II, and X, aggrecan, osteopontin, and osteonectin (by reverse transcription-polymerase chain reaction) and collagen types I and II (by immunohistochemistry). Reactive tissue was present in 93% of cases. Histologically, this tissue consisted of hyaline cartilage at follow-up days 10 and 20. Expression of collagen type II and aggrecan was present at 10 and 20 days postsurgery. Highest expression was at 10 days. Expression of collagen type X increased up to 20 days. No significant changes in the mRNA expression of osteopontin or osteonectin were observed. Immunohistochemistry confirmed the presence of cartilage, which was positive for collagen types I and II at 10 days and only for collagen type II at 20 days. At 20 days postsurgery the onset of bone formation was also observed. At 40 days postsurgery, the reactive tissue had almost completely turned into bone. The quality and amount of cartilage formed 10 days postsurgery make this technique potentially useful to fill large cartilage and bone defects. Also, periosteal callus formation, providing possible useful information for tissue engineering techniques, can be studied with this model.
Personal Analysis, Interpretation & Implication:
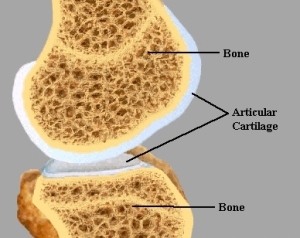 What we are seeing is that the researchers state quite explicitly that “damaging the periosteum may be a way to generate ectopic cartilage or bone”. If we now remember, one of the central ideas on why Tyler thinks the LSJL technique works is because it leads to ectopic (not in the normal impacted location) microfractures which can result in mini growth plates. In the experiment the researchers cut into the bone on the proximal medial tibia side, beyond past the periosteum and got to the layer of proliferative cells right underneath the periosteum which is what really leads the bones to develop width based appositional periosteal growth leading to an overall constant thickness in the outer bone area/volume.
What we are seeing is that the researchers state quite explicitly that “damaging the periosteum may be a way to generate ectopic cartilage or bone”. If we now remember, one of the central ideas on why Tyler thinks the LSJL technique works is because it leads to ectopic (not in the normal impacted location) microfractures which can result in mini growth plates. In the experiment the researchers cut into the bone on the proximal medial tibia side, beyond past the periosteum and got to the layer of proliferative cells right underneath the periosteum which is what really leads the bones to develop width based appositional periosteal growth leading to an overall constant thickness in the outer bone area/volume.
The periosteal tissue is taken at certain days after the cut made past the periosteum into the harvest tissue layer (day 10, 20, and 30). When these tissue is tested looking at the types of cells that develop after a few days, the researchers noticed that the periosteal tissue first turns into cartilage. This is confirmed from an immunohistochemical measurement where the fact that Collagen type I and type II are both expressed from the tissue the most around the 10 days postsurgery point. After a few more days however, the cartilage appear to change into bone cells from noticing the increased mRNA expression of collagen type X.
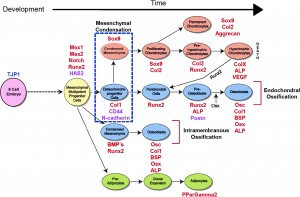
This indicates that it might be possible through delaying or suspending the inner cambium layer of the periosteum to cause a layer of cartilage to form and that can be converted into a chondrocyte stack leading to longitudinal legnthening.
Clin Orthop Relat Res. 1999 Oct;(367 Suppl):S186-203.
Articular cartilage regeneration using periosteum.
Source
Department of Orthopedic Surgery, Mayo Clinic, Mayo Foundation, Rochester, MN 55905, USA.
Abstract
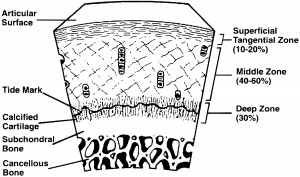
Periosteum has chondrogenic potential that makes it possible to repair or regenerate cartilage in damaged joints. Whole periosteal explants also can be cultured in vitro for the purpose of studying chondrogenesis. This chondrogenic potential arises because the cambium layer of periosteum contains chondrocyte precursor cells that form cartilage during limb development and growth in utero, and does so once again during fracture healing. The advantages of whole tissue periosteal transplants for cartilage repair include the fact that this tissue meets the three primary requirements for tissue engineering: a source of cells, a scaffold for delivering and retaining them, and a source of local growth factors. Data from in vivo studies show that periosteum transplanted into osteochondral articular defects produce cartilage that can restore the articular cartilage and be replaced by bone in the subchondral region. This capacity is determined by surgical factors such as the orientation of the cambium layer, postoperative factors such as the use of continuous passive motion, and the age and maturity of the experimental animal. In vitro studies have shown that the chondrogenic potential of periosteal explants is determined by culture, donor conditions, and technical factors. Chondrogenesis is optimized by suspension of the explants in agarose under aerobic conditions, with supplementation of the media using fetal calf serum and growth factors, particularly transforming growth factor-beta 1. The role of physical factors currently is being investigated, but studies show that the mechanical environment is important. Donor factors that are important include the harvest site, the size of the periosteal explant, and most importantly the age of the donor. Periosteal chondrogenesis follows a specific time course of events, with proliferation preceding differentiation. The current challenge is to clarify the process of periosteal chondrogenesis and its regulation at the cellular and molecular levels, so that it can be controlled intelligently and optimized for the purpose of cartilage repair and regeneration.
{Here’s an article I found-Tyler
Elucidating multiscale periosteal mechanobiology: a key to unlocking the smart properties and regenerative capacity of the periosteum?
“The periosteum, a thin, fibrous tissue layer covering most bones, resides in a dynamic, mechanically loaded environment. The periosteum also provides a niche for mesenchymal stem cells. Periosteum exhibits stress-state-dependent mechanical and material properties, hallmarks of a smart material.”
” In absence of graft, infilling occurs from the periosteum, toward the surface of the implant, which stabilizes the femur and fills the medullary cavity. Biophysical and chemical environment of PDCs egressing from the periosteum into the critical-sized defect modulates tissue genesis (chondro- as well as osteogenesis).”
“the cytoskeleton is akin to a living bridge that restructures its architecture to minimize areas of stress concentration in high wind or traffic situations. Similarly, at the length scale of a tissue, the periosteum serves as bone’s bounding membrane and harnesses endogenous biophysical cues to modulate environmental conditions on either side (within and outside of bone).”
“Periosteum is highly vascularized and provides at least 1/3 of the blood supply to cortical bone, with the remaining supply coming from the intramedullary niche.”
“The periosteum is anchored to the bone by Sharpey’s fibers, strong fibers with a high collagen content. Sharpey’s fibers serve as a link between the exterior musculature and the interior skeleton and allow the periosteum to remain intact and attached to the bone, even after severe trauma occurs. In certain bones, Sharpey’s fibers anchor tendons and ligaments to the bone. Periosteum is absent at sites of tendon attachments. As tendon and ligament attachments vary by bone, periosteum morphology is highly variable between bones and even within bones.”
 “PDCs[Periosteum Derived Stem Cells] and BMSCs have been shown to differentiate along different lineages when cultured on the same roughened titanium surface, suggesting different pathways or mechanisms for mechanotransduction for MSCs from different niches.”
“PDCs[Periosteum Derived Stem Cells] and BMSCs have been shown to differentiate along different lineages when cultured on the same roughened titanium surface, suggesting different pathways or mechanisms for mechanotransduction for MSCs from different niches.”
“The native environment of PDCs is mechanically regulated by a combination of tension and shear (given that the periosteum itself exhibits different moduli of elasticity in the longitudinal and circumferential directions). The intracellular tension PDCs experience is suggested to regulate long bone growth.”
“PDCs’ capacity to carry intracellular tension through their active microfilament network has been postulated to regulate a signaling cascade which, in turn, is responsible for the expression of soluble factors that modulate cartilage growth. The stiffness of the culture surface determines the magnitude of intracellular tension placed on the actin microfilament network.”
“mechanical signaling alone can cause PDCs to differentiate”
“PDC proliferation during periosteal chondrogenesis can be stimulated by DFP[Dynamic Fluid Pressure].”}
- PMID: 10546647 [PubMed – indexed for MEDLINE
- Analysis and Interpretation: The abstract shows that the cambium layer of periosteum has the precursor to chondrogenic type cells. These pregenitor cells are what form the cartilage in limb development in growth. They are also what caused the callus formation from fracture healing. Apparently the scientists have already been using this type of cell for tissue transplants for at least articular cartilage repair since it satisfies all three requirement, being a source of cells acting also as a scaffold and also hace local growth factors. It seems that the ability for transplanted and implanted periosteal tissue is determined by the culture, the donor conditions, and technical factors. However, we note that all of these factors can be manipulated in the laboratory until the optimum conditions for the three factors are found. The researchers in turn understand very well which factors are critical and which factors will need to be tested to see they are at the optimum conditions. The ending of the abstract shows that the technology for using periosteum derived pre-cursor chondrocytes is under way. they state “The current challenge is to clarify the process of periosteal chondrogenesis and its regulation at the cellular and molecular levels, so that it can be controlled intelligently and optimized for the purpose of cartilage repair and regeneration.”
-
The role of periosteum in cartilage repair.
Clin Orthop Relat Res. 2001 Oct;(391 Suppl):S190-207.
The role of periosteum in cartilage repair.
O’Driscoll SW, Fitzsimmons JS.Source
Department of Orthopedic Surgery, Mayo Clinic, Mayo Foundation, Rochester, MN 55905, USA.
Abstract
Periosteum, which can be grown in cell and whole tissue cultures, may meet one or more of the three prerequisites for tissue engineered cartilage repair. Periosteum contains pluripotential mesenchymal stem cells with the potential to form either cartilage or bone. Because it can be transplanted as a whole tissue, it can serve as its own scaffold or a matrix onto which other cells and/or growth factors can be adhered. Finally, it produces bioactive factors that are known to be chondrogenic. The chondrocyte precursor cells reside in the cambium layer. These vary in total density and volume with age and in different donor sites. The advantages of whole tissue periosteal transplants for cartilage repair include the fact that this tissue meets the three primary requirements for tissue engineering: a source of cells, a scaffold for delivering and retaining them, and a source of local growth factors. Many growth factors that regulate chondrocytes and cartilage development are synthesized by periosteum in conditions conducive to chondrogenesis. These include transforming growth factor-beta 1, insulinlike growth factor-1, growth and differentiation factor-5, bone morphogenetic protein-2, integrins, and the receptors for these molecules. By additional study of the molecular events in periosteal chondrogenesis, it may be possible to optimize its capacity for articular cartilage repair.
Analysis and Interpretation: This abstract is a sort of review of the technology to use periosteum in cartilage repair. It has one or more of the three primary factors to make tissue engineered cartilage repait possible. It has the pluripotent mesenchymal stem cells, it has the scaffold or matrix which the cells and growth factors can adhere to. It also produces within itself (locally) the type of growth factors need to lead the MSCs to cartilage cells. The growth factor the periosteum can manufacturer and release for cartilage formation are TGF-beta 1, IGF-1, GDF-4, BMP-2, integrins, and the receptor for the growth factors. While the writers of this article are imagining and thinking abut how to use this pool of amazing resource for articular cartilage repair, we are thinking about how to use the periosteum in epiphyseal cartilage regeneration.
- PMID: 11603704 [PubMed – indexed for MEDLINE]

based on my own results I estimate LSJL growth rate is (0-3mm) per month with possible growth spurts of up to 1/2 inch / over several years if performed to its fullest potential i have hit full 5ft 10 inch morning height and my evening is a strong 177cm i have gained close to an inch in height .I used to mark my evening height at /175 mark .it does work only in the long term slowly its has a similar to bodybuilding comparsion adult height building is far more difficult and complex than that.with regards i think possible short man genetics could possibly impair LSJL from working at all similar to skinny hard gainers that could not gain muscle .