“[The growth plate] functions to produce a mineralised cartilaginous scaffold to which new trabecular bone is formed via a tightly controlled two-step process (called endochondral ossification) involving chondrogenesis and osteogenesis ”
“The resting zone has previously been thought to play a very minimal role during endochondral ossification as the pre-chondrocytes/cells within this zone proliferate minimally. However, studies have indicated the importance of the resting zone as it acts as a reservoir of stem cells/pre-chondrocytes for the chondrocytes in the adjacent proliferative zone”
“The proliferative zone is responsible for matrix production (including collagen-2 and aggrecan) and cellular division during endochondral ossification. The height of the proliferative zone directly correlates with the extent of longitudinal growth that can be achieved by the long bone. As regulated by various signalling pathways including parathyroid hormone-related protein, insulin-like growth factor (IGF1), bone morphogenic protein (BMP), Wnt/B-catenin, fibroblast growth factor (FGF) and others , chondrocytes cease to proliferate and become hypertrophic. The hypertrophic chondrocytes produce collagen-10 which is involved with matrix mineralisation. Together with the action of angiogenic factor vascular endothelial growth factor (VEGF) produced by hypertrophic chondrocytes and a low oxygen tension, the lower hypertrophic zone attracts blood vessel invasion from the adjacent metaphyseal bone, which brings along mineralised cartilage-resorptive cells (chondroclasts), bone-forming cells (osteoblasts) and bone-resorptive cells (osteoclasts) to convert the mineralised cartilage scaffold into trabecular bone in metaphysis.”
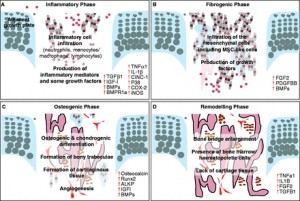
“The initial inflammatory response involves an influx of key inflammatory cells into the growth plate injury site and up-regulation of inflammatory cytokines/mediators and some growth factors (A). The fibrogenic phase involves an influx of fibrogenic and progenitor cells containing MSC-like cells (B). The osteogenic phase involves the osteogenic and chondrogenic differentiation, formation of bony trabeculae together with angiogenesis within the injury site (C). The remodelling phase involves the maturation and active remodelling of the newly formed bony trabeculae as well as disappearance of cartilaginous repair tissue (D).”<-Cinc1 is also known as IL8 or CXCL1{Which is upregulated by LSJL}
“neutrophil-mediated inflammatory response was found to modulate downstream injury repair events. Following the depletion of neutrophils with a neutralising antibody, an increase in the undesirable bony repair tissue [occurred] with increased expression in bone-related genes such as Runx2 and osteocalcin, but decreased expression in cartilage-related genes Sox9 and collagen-2 ”
“Blocking TNFa resulted in a clear delay in the subsequent mesenchymal infiltration response and a reduction of the proliferation of these cells”
“At the growth plate injury site, some of these cells were found to express growth factors including BMPs, platelet-derived growth factor (PDGF) and FGF2 and receptors for BMPs and PDGF. In addition, some of these cells were found to be MSC like as they expressed the stem cell marker alpha-smooth muscle actin{acta2 which is upregulated by LSJL}”
“this influx of mesenchymal cells may contain a myriad of cells including MSC-like cells, osteoprogenitor cells, pre-osteoblasts, and/or pre-chondroblasts (either pre-existing or newly derived from the infiltrated MSCs).”
“significant peak in the mRNA expression of platelet-derived growth factor (Pdgf) and fibroblast growth factor 2 (Fgf2){up} following the initial inflammatory phase, suggesting a potential regulatory role for these two growth factors during this phase”
“In rats with growth plate injury, the inhibition of PDGF signalling caused a significant reduction in the amount of mesenchymal infiltrate, decreased amounts of bony and/or cartilage repair tissues, and thus an overall delay in bony repair 14 days post-injury”
“At the injured growth plate, bone formation has been observed to commence around day 7 with the appearance of bony trabeculae, and bone remodelling has been observed by day 14 with the appearance of bone marrow cells in between bony trabeculae”
“During the osteogenic phase of the growth plate injury repair process, the cells within the fibrogenic infiltrate differentiate into Runx2 and alkaline phosphatase-immunopositive osteoblasts and produce increased levels of bone matrix protein osteocalcin (both mRNA and protein) during days 8–14”
“after a ‘fibrous tissue’ is formed from the infiltrated stromal cells at an injured growth plate, its invasion by new blood vessels is a prerequisite for its osseous transformation”
“the absence of VEGF delayed bone formation by halting the initial soft callus from being converted into hard bony callus. ”
“osterix over-expression can induce bone healing”
“PKD up-regulates osterix and [is] important for osteoblast differentiation. Inhibition of PKD suppressed bony repair but induced more chondrogenic differentiation at the injury site”
“over-expression of osterix in osteochondroprogenitor cells resulted in a decrease in chondrogenic transcription factor Sox9.”
“In a rat tibial drill hole growth plate injury model, levels of Bmp2 mRNA expression were found notably increased in the early part of the fibrogenic phase and then again later during the osteogenic phase”
“synovium-derived MSCs in particular had the greatest capability to enhance the chondrogenic differentiation potential when compared with any other mesenchymal tissue-derived cells. However, other studies have reported that bone marrow-derived MSCs (BMMSCs) are most suitable for cartilage tissue engineering, as they possess higher proliferation rates and higher levels of expression of cartilage-specific genes, when compared with MSCs derived from other tissues”
“MSCs were successfully isolated directly from murine epiphysis. This novel type of MSCs could potentially be better than BMMSCs as they have shown greater capacities in growth and differentiation potential as well as possessing immunosuppressive and anti-inflammatory properties”