Growth plate cellular senescence preceeds epiphyseal fusion. It is the reason why people stop growing. If we stop cellular senescence we can keep growing. And this study states that stem cell growth is not based on demand. Demand for stem cells too high to be kept up with may lead to cancer growth. However, supplements, reduction of environmental stressors, and mechanical loading may be some ways to stimulate stem cell health and renewal. The key to LSJL success on restoring growth renewal may be based on it’s ability to stimulate stem cell proliferation and impair cellular senescence. As this study suggests that microcracks that occur during old age increase the demand for stem cells but the body doesn’t generate new stem cells or increase proliferation to compensate. However, unlike microcracks due to degradation LSJL places forces directly on the cells themselves. I will be posting some updates on the LSJL method as soon as the comments section is up(after Michael has resolved some legal issues).
“Knudson’s carcinogenic model, which simulates incidence rates for retinoblastoma, provides compelling evidence for a two-stage mutational process. However, for more complex cancers, existing multistage models are less convincing. To fill this gap, I hypothesize that neoplasms preferentially arise when stem cell exhaustion creates a short supply of progenitor cells at ages of high proliferative demand. To test this hypothesis, published datasets were employed to model the age distribution of osteochondroma, a benign lesion, and osteosarcoma, a malignant one. The supply of chondrogenic stem-like cells in femur growth plates of children and adolescents was evaluated and compared with the progenitor cell demand of longitudinal bone growth. Similarly, the supply of osteoprogenitor cells from birth to old age was compared with the demands of bone formation. Progenitor cell demand-to-supply ratios are a good risk indicator, exhibiting similar trends to the unimodal and bimodal age distributions of osteochondroma and osteosarcoma, respectively. The hypothesis also helps explain Peto’s paradox and the finding that taller individuals are more prone to cancers and have shorter lifespans. The hypothesis was tested, in the manner of Knudson, by its ability to convincingly explain and demonstrate, for the first time, a bone tumour’s bimodal age-incidence curve.”
“Osteochondroma is the most common benign bone tumour, occurring as an abnormal osteocartilaginous outgrowth of the epiphyseal growth plate with a low rate (≤2%) of malignant transformation to secondary chondrosarcoma”
“In adolescents, growth plate senescence is followed by epiphyseal fusion when osteochondroma stop growing.”
“Osteosarcoma is the most common primary bone malignancy (excluding multiple myeloma), originating from the transformation of aberrant bone-forming mesenchymal stem cells (MSC), also known as marrow stromal cells”
“Differentiation is impaired by replicative senescence”
“Post-natal stem cell replication leads to telomere shortening in somatic tissues, as telomerase activity in humans is not at sustaining levels. At the Hayflick limit of cell division, telomeres reach a critical length, triggering cells to become senescent or apoptotic. A recent publication provides quantitative evidence that soft tissue organ mass loss in humans aged 25–70 is significantly associated with the log of shorter cell turnover times, implicating stem cell exhaustion and replicative senescence in normal ageing”
“MSC, like other stem cells, have been observed to have a self-renewal and proliferative capacity that diminishes with age, and that is regulated by different pathways, such as antioxidant defence, DNA repair, and protein turnover, before entering a senescence-associated proliferation arrest”
“There is a normal trade-off for a stem cell to self-renew or produce differentiated and differentiating progeny. High stem cell demand relative to supply appears to produce stem cell progeny with stopped differentiation but with self-renewal properties, perhaps triggered by senescent cell secretions”
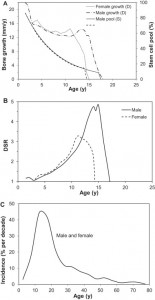
Here’s a graph describing the stem cell pool:
“A good marker of the skeletal demand for osteoprogenitors involved in bone formation (as opposed to osteoclast bone resorption activity) is serum BALP activity. This marker is particularly sensitive to physiological changes such as adolescent growth spurt and menopause. The demand for progenitor cells participating in bone formation, as measured by BALP activity, is highest in newborns, slowly declines until early adolescence in girls and late adolescence in boys, then decreases rapidly to its lowest level in young adults, before increasing again in the latter half of adulthood to cope with the decline in osteocyte density and repair of accumulated damage related to ageing, e.g. bone microcracks”
“senescent cells can arise due to “epigenetic senescence” (initiated by histones altering activity of genes), stress-induced senescence or replicative senescence”<-Stress-induced sensescence is the easiest to avoid and epigenetic senescence can be affected by supplements. Replicative senescence may be influenced by mechanical loading.
“cell proliferation is not in itself a risk factor for tumogenesis; instead, neoplastic promotion or progression occurs preferentially when, for example, stem cell exhaustion creates a short supply of progenitor cells at ages of high mitogenic demand.”
” Ionizing radiation exposures produce free radicals and reactive oxygen species that excite a high proliferative demand on MSC involved in inflammation and subsequent fibrosis. When bone marrow is subjected to a high dose, there is an increase in expression of the ageing and senescent cell biomarker p16INK4a reducing the capacity for stem cell renewal and, consequently, causing a decline in cellularity commensurate with premature ageing”