This is a study that could have massive ramifications on height increase but the the exact mechanism is unknown. But that cells that have the genetic material of growth plate cells are retained in adult bone is huge news.
Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation.
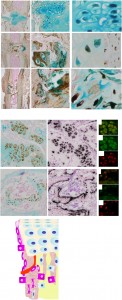
hypertrophic chondrocytes<-full study
“Chondrocytes and osteoblasts are considered independent lineages derived from a common osteochondroprogenitor. In endochondral bone formation, chondrocytes undergo a series of differentiation steps to form the growth plate, and it generally is accepted that death is the ultimate fate of terminally differentiated hypertrophic chondrocytes (HCs). Osteoblasts, accompanying vascular invasion, lay down endochondral bone to replace cartilage. [Can] HC become an osteoblast and contribute to the full osteogenic lineage? Here we use a cell-specific tamoxifen-inducible genetic recombination approach to track the fate of murine HCs and show that they can survive the cartilage-to-bone transition and become osteogenic cells in fetal and postnatal endochondral bones and persist into adulthood. This discovery of a chondrocyte-to-osteoblast lineage continuum revises concepts of the ontogeny of osteoblasts, with implications for the control of bone homeostasis and the interpretation of the underlying pathological bases of bone disorders.”
This is huge because it means that the transdifferentiated osteoblasts retain some of the chondrocytic genetic material which means they could possibly dedifferentiate back into chondrocytes!
“The expression of preosteoblastic markers in LHs before the formation of the POC raises the possibility that these cells may transition to an osteoblastic fate”
“HC-Derived Cells Are Present in Fetal, Neonatal, and Adult Bone.”
“The HC-derived cells, morphologically resembling osteoblasts, were found close to the chondro-osseous junction, on the surface of trabeculae, and in the endosteum”<-that means they are in a good position to be involved in neo growth plate formation.
“HC[hypertrophic chondrocytes] Derivatives Transit to the Primary Spongiosa and Become Col1a1-Expressing Cells.”<-the majority of HC derivatives become osteoblasts.
“HC-to-bone transition occurs during postnatal bone growth and that HC-derived cells may be long-lived within the mature bone”
Here’s an image of the HC lineage:
“The reversion of HCs to a prehypertrophic- like state in response to endoplasmic reticulum (ER) stress suggests that hypertrophy is not an irreversible state in vivo”<-Could we revert HC-derived osteoblasts back to hypertrophic chondrocytes back to pre-hypertrophic cells to reform growth plates.
(Michael: That actually makes a lot of sense if one thought about it. It can’t be all dead inorganic bone ECM matter where the physeal cartilage turns into bones (aka Primary Spongiosa). How would bone cells even be able to travel to that middle-region later on if the chondrocytes completely died out and the lucanae was covered by calcium minerals. For the smooth gradual transition from the hypertrophic layer to the vascularization/calcification layer to the mineralization layers to work out, there would be some minority of chondrocytes which would change identities. If all the chondrocytes died out and the layer of the lucanae was completely mineralized, I would not be sure that osteoblasts would be able to reach the primary spongiosa layer.
Here is what I have found years ago. (Source: Chondrogenesis just ain’t what it used to be) Prehypertrophic chondrocytes secrete IHH while the chondrocytes from the perichondrium secrete PTHrP, which effect the PTH/PTHrP receptors which are on the prehypertrophic chondrocytes. In addition, we have to consider the effects of the VEGF. VEGF is secreted by hypertrophic chondrocytes, and that it acts as a major inducer of vascular invasion.
The big thing is the following…
“…demonstrated that Ihh is not only required for chondrocyte hypertrophy, but also for expression of Cbfa1, a transcription factor required for osteoblast differentiation”
If the molecular biologist researchers have been able to identify all of the major players which causes the chondrocytes to go into apoptosis, and also transdifferentiation, then we at least know which transcription factors causes the changes. I suspect it is Cbfa1.
Refer also the seminal work “Indian hedgehog couples chondrogenesis to osteogenesis in endochondral bone development”
That study proved conclusively that it is IHH that starts everything. IHH stimulates the Cbfa1, which causes the vascularization. I am going to make a guess that somewhere along the way, the two peptides also caused the transdifferentiation.
So how do we actually figure out what type of chemical would lead the osteoblast/osteocytes types to go in reverse and de-differentiate aka some type of reverse transdifferentiation?
We first have to assume that there is some type of external stimuli (mechanical, chemical, electrical) which would be able to even do the de-differentiation.
I propose this idea for Tyler…
Let’s assume that if we change the environment, the cell will start to change its identity. Remember the study which showed that the path which the MSCs will differentiate into can be determined by the shape of space that they are placed into? That suggest that if we change the environment that the osteoblasts/bone cells are in, maybe they will change as well.
Here is my first idea: I propose that we try to remove the calcium crystals/de-mineralize the area. To do that, remember that the PTH and the PTHrP balance from the parathyroid glands controls the level of calcium that is dissolved into the human blood (refer to “Chapter 5 – The parathyroid glands and vitamin D“)
“In bone, within 1 or 2 hours, PTH stimulates a process, known as osteolysis, in which calcium in the minute fluid-filled channels (canaliculi/lacunae) is taken up by syncytial processes of osteocytes and transferred to the external surface of the bone and, thence, into the extracellular fluid. Some hours later, it also stimulates resorption of mineralized bone; a process that releases both Ca2+ and Pi into the extracellular fluid. The Pi is rapidly removed from the circulation because the most dramatic effect of PTH on the kidney is to inhibit reabsorption of Pi in the proximal tubule and markedly increase its excretion“
Let’s increase the level of PTHrP in the local region, which is what I had proposed in a post I had written more than a year ago (The Connection Between Regenerating Deer Antlers and The PTHrP, PTH And IHH pathway for Cartilage Regulation, PTHrP Seems To Be The Answer (Big Breakthrough!)). Decrease the concentration of CA2+ from the ECM, and see what happens to the osteoblasts. Would they de-differentiate into chondrocytes if their environment changes?
It might be that the formation of osteoblasts and the increased levels of mineralization/vascularization/calcification is a positive feed back loop where each part feeds upon itself, which is initialized by VEGF. If we break the positive cycle at the process of mineralization, would the osteoblasts also go in reverse?

Pingback: New info about reversing growth plate ossification: chondrocyte transdifferentiation into osteoblasts - Natural Height Growth
So could this lead to developments in increasing height?
Of course. The question is how to get scientists to act upon it.