Note: A lot of the material and information that I will be posting on this post will also be found on the “Protein/Hormone Signal Pathway Map” Post and also the “Molecular Biology, Biochemistry” section of the website
Note: As like the PI3K/AKT pathway, there is a video you can watch to help make the learning process a little easier from YouTube HERE.
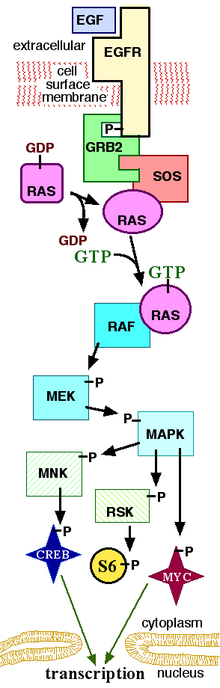
From the wikipedia article on MAPK/ERK pathway HERE.. a simplified version of the MAPK pathway is available below.
The MAPK/ERK pathway is a chain of proteins in the cell that communicates a signal from a receptor on the surface of the cell to the DNA in the nucleus of the cell. The signal starts when a signaling molecule binds to the receptor on the cell surface and ends when the DNA in the nucleus expresses a protein and produces some change in the cell, such as cell division. The pathway includes many proteins, including MAPK (originally called ERK), which communicate by adding phosphate groups to a neighboring protein, which acts as an “on” or “off” switch. When one of the proteins in the pathway is mutated, it can be stuck in the “on” or “off” position, which is a necessary step in the development of many cancers. Components of the MAPK/ERK pathway were discovered when they were found in cancer cells. Drugs that reverse the “on” or “off” switch are being investigated as cancer treatments.
The pathway
Overall, the extra-cellular mitogen binds to the membrane ligand. This allows Ras (a GTPase) to swap its GDP for a GTP. It can now activate MAP3K (e.g., Raf), which activates MAP2K, which activates MAPK. MAPK can now activate a transcription factor, such as myc.
Coupling cell surface receptors to G proteins
Receptor-linked tyrosine kinases such as the epidermal growth factor receptor (EGFR) are activated by extracellular ligands. Binding of epidermal growth factor (EGF) to the EGFR activates the tyrosine kinase activity of the cytoplasmic domain of the receptor. The EGFR becomes phosphorylated on tyrosine residues. Docking proteins such as GRB2 contains an SH2 domain that binds to the phosphotyrosine residues of the activated receptor. GRB2 binds to the guanine nucleotide exchange factor SOS by way of the two SH3 domains of GRB2. When the GRB2-SOS complex docks to phosphorylated EGFR, SOS becomes activated. Activated SOS then promotes the removal of GDP from a member of the Ras subfamily (most notably H-Ras or K-Ras). Ras can then bind GTP and become active.
Apart from EGFR, other cell surface receptors that can activate this pathway via GRB2 include Trk A/B, Fibroblast growth factor receptor (FGFR) and PDGFR.
Kinase cascade
Activated Ras activates the protein kinase activity of RAF kinase. RAF kinase phosphorylates and activates MEK (MEK1 and MEK2). MEK phosphorylates and activates a mitogen-activated protein kinase (MAPK).
RAF, and MAPK are both serine/threonine-selective protein kinases. MEK (also known as MAPKK) is a tyrosine/threonine kinase.
In the technical sense, RAF, MEK, and MAPK are all mitogen-activated kinases, as is MNK. MAPK was originally called “extracellular signal-regulated kinases” (ERKs) and “microtubule-associated protein kinase” (MAPK). One of the first proteins known to be phosphorylated by ERK was a microtubule-associated protein (MAP). As discussed below, many additional targets for phosphorylation by MAPK were later found, and the protein was renamed “mitogen-activated protein kinase” (MAPK). The series of kinases from RAF to MEK to MAPK is an example of a protein kinase cascade. Such series of kinases provide opportunities forfeedback regulation and signal amplification.
Regulation of translation and transcription
Three of the many proteins that are phosphorylated by MAPK are shown in the Figure. One effect of MAPK activation is to alter the translation of mRNA to proteins. MAPK phosphorylates 40S ribosomal protein S6 kinase (RSK). This activates RSK, which, in turn, phosphorylates ribosomal protein S6.[5] Mitogen-activated protein kinases that phosphorylate ribosomal protein S6 were the first to be isolated.[4]
MAPK regulates the activities of several transcription factors. MAPK can phosphorylate C-myc. MAPK phosphorylates and activates MNK, which, in turn, phosphorylates CREB. MAPK also regulates the transcription of the C-Fos gene. By altering the levels and activities of transcription factors, MAPK leads to altered transcription of genes that are important for the cell cycle.
The 22q11, 1q42, and 19p13 genes are associated with schizophrenia, schizoaffective, bipolar, and migraines by affecting the ERK pathway.
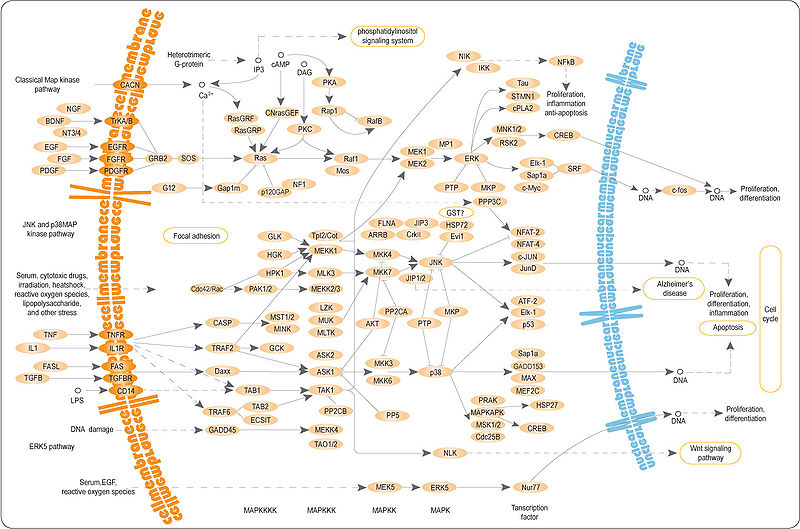
From wikipedia link on MAPK/ERK Pathway HERE, I will post a picture of the pathway below for analysis
Analysis & Interpretation:
This pathway has an overall similar structure and area of function as the Wnt/Beta-Catenin Signal Pathway as we have seen before. It is a protein chain or cascade signal pathway where the chain of protein interactions start off at the outer surface of the cell, in the plasma membrane. The outer receptors on the bi-lipid layer gets attached to what is known as a signal molecule. The end result is that the DNA is affected in a certain way from a multiple of cascading signalling pathways to produce or cause a certain type of protein to be either increased or decreased in number, which will affect the overall cell.
The effect on the cell can be just as that it will be told by the proteins produced to focus mainly on differentiation, replication, division, proliferation, apoptosis, etc.
The reason the pathway is called the MAPK/ERK pathways is because one of the more major components in this cascade or chain is the MAPK which used to be called the ERK. The MAPK stands for Mitogen-Activated Protein Kinase and the ERK stands for Extracellular-Signal-Regulated Kinases. Like most other protein kinases, their job is to add on a phosphate group to some protein that is close by to it in the intracellular matrix to turn it either “on” or “off”.
Overall there is one major overall pathway or cascades going on, with many smaller more individual pathways all happening.
1. Extracellular mitogen binds to the membrane ligand –> Something called a RAS changes the GDP its has for a GTP –> This actives the a type of protein kinase group called MAP3K (one of the proteins in the MAP3K group is RAF) to signal and activate the kinase group MAP2K which signals and activates the kinase group MAPK. The protein kinase group MAPK ultimate can effect transcription factors like MYC (have no idea what that is right now).
- The membrane ligand or membrane receptor can be like a epidermal growth factor receptor (EGFR). These receptors are linked beforehand to another kinase.
- The extracellular mitogen can be like a epidermal growth factor (EGF). Another name for the extrecellular mitogen is the extracellular ligands
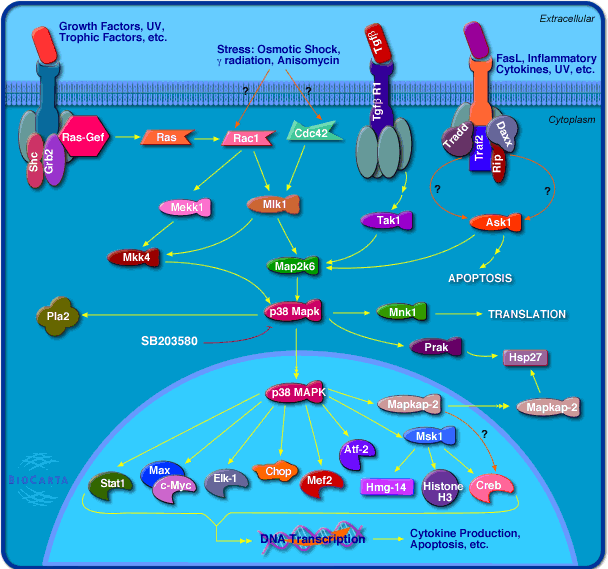
The result is that there will be some types of residues formed from the activation of the receptor linked to the kinase. Different types of proteins which then dock on the kinase will get phosphorylated and this will in effect activate other proteins around it. From the diagram below we can see that at some stage of the multiple cascading web of protein signal pathways, the p38 MAPK protein kinase will have an effect on around 10 other neighboring proteins and compounds.
At this point, I have decided to end my detailed study on the MAPK/ERK signaling pathway here because the details I feel at not important at this stage of the research. Maybe later at some point this post and my knowledge on signaling pathways will be needed to be updated and improved upon.
From the Biocarta website I found another picture for analysis (source HERE) below…
{Tyler-Related Paper:
ERK1 and ERK2 regulate chondrocyte terminal differentiation during endochondral bone formation.
“Chondrocytes in the epiphyseal cartilage undergo terminal differentiation prior to their removal through apoptosis. To examine the role of ERK1 and ERK2 in chondrocyte terminal differentiation, we generated Osterix (Osx)-Cre; ERK1(-/-) ; ERK2(flox/flox) mice (conditional knockout Osx [cKOosx]), in which ERK1 and ERK2 were deleted in hypertrophic chondrocytes. These cKOosx mice were grossly normal in size at birth, but by 3 weeks of age exhibited shorter long bones. Histological analysis in these mice revealed that the zone of hypertrophic chondrocytes in the growth plate was markedly expanded. In situ hybridization and quantitative real-time PCR analyses demonstrated that Matrix metalloproteinase-13 (Mmp13) and Osteopontin expression was significantly decreased, indicating impaired chondrocyte terminal differentiation. Moreover, Egr1 and Egr2, transcription factors whose expression is restricted to the last layers of hypertrophic chondrocytes in wild-type mice, were also strongly downregulated in these cKOosx mice. In transient transfection experiments in the RCS rat chondrosarcoma cell line, the expression of Egr1, Egr2, or a constitutively active mutant of MEK1 increased the activity of an Osteopontin promoter, whereas the MEK1-induced activation of the Osteopontin promoter was inhibited by the coexpression of Nab2, an Egr1 and Egr2 co-repressor. These results suggest that MEK1-ERK signaling activates the Osteopontin promoter in part through Egr1 and Egr2. Finally, our histological analysis of cKOosx mice demonstrated enchondroma-like lesions in the bone marrow that are reminiscent of human metachondromatosis, a skeletal disorder caused by mutations in PTPN11. Our observations suggest that the development of enchondromas in metachondromatosis may be caused by reduced extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK MAPK) signaling.”