Low-Intensity Pulsed Ultrasound LIPUS Does Not Increase Longitudinal Growth Of Bone
Update March 6th 2014: Tyler did leave a response to this post originally but there is now even more evidence found which tips the question of the effect of Low-Intensity Pulsed Ultrasound on bone longitudinal growth to the side of having no affect at all.
Study #1: Effects of therapeutic ultrasound on longitudinal growth of the femur and tibia in rats
The conclusion for this particular study was that using the three different intensities of LIPUS at 0.5, 1.0 W/cm2, and 1.5 W/cm^2 it was found that the emissions had no effect. There was no inhibitory effect and there was no stimulatory effect. If we were to be extremely accurate, there was a very slight stimulatory effect in the first two groups and an inhibitory effect in the third group, but the slight deviations were too small to be considered significant.
There have been some studies like the Chapter 5 “Low-intensity ultrasound stimulates endochondral ossification in vitro” which shows that using LIPUS is critical when going through the beginning stages of endochondral ossification though.
This recent news is slightly sad to hear but this is one study I did find by Joseph Spadaro, who collaborated with Dr. Robert Becker about 30 years ago, which shows that the LIPUS technology which we had previously believed to work in increasing bone growth (at least density wise) seems to do very little towards the longitudinal growth of bones “Application of low-intensity ultrasound to growing bone in rats.”
Abstract Below
Low-intensity pulsed ultrasound recently has been shown to accelerate long bone fracture healing, but its effect on bone growth and development is unknown. The longitudinal growth and bone density of the femur and tibia in young rats was measured after application of an ultrasound transducer emitting 1.5-MHz pulsed ultrasound (30 mW/cm2, SATA) for 20 min/day. After 28 days, no length difference was detected (< or = 2%) compared to the sham-treated leg or to unexposed controls. Also, no significant difference in bone mineral density (BMD) of the femur or tibia was found (< or = 6%). In a repeated experiment in which a periosteal trauma stimulus was created in the femoral diaphysis, the ultrasound also had no effect on growth or BMD. This results suggests that physeal bone growth is far less sensitive to this level of ultrasound application than is fracture repair. This may be related to the cascade of cellular events and regulatory factors that are present after a fracture.
So what does this mean for us?
Recently I have been looking into the piezoelectric properties of the bone, as described by the book The Body Electric. Dr. Becker wrote that back in the 1975-1980 year range, he had found that from the arrangement of how the collagen fibers and apatite crystals are aligned next to each other, they created a PN Junction as well as being piezoelectric. The bones in our body are actually semi-conductors.
While the research group originally thought that the apatite, being a crystal, would be piezoelectric, it turned out that the large collagen fibers seemed to be the real compound.
The author of this paper was one of his colleagues, whom he collaborated extensively with. I’ve searched through the PubMed database to see what he has written in the decades that followed. This particular study done by the current professor emeritus of at SUNY suggest that even using young lab rats (which means that they have healthy growth plates available), the LIPUS technology of placing a transducer emitting Ultrasound at levels of 1.5-MHz pulsed ultrasound (30 mW/cm2, SATA) for 20 minutes every day.
If the transducer has no effect on young rats with clearly available cartilage to possibly stimulate, then it would most likely not have any effect on humans with no cartilage at all.
Note the following points made
- There was no effect on longitudinal growth of the leg bones (femur and tibia) after 30 days
- There was no effect on the bone mineral density of the same bones after 30 days.
- When a load was tried on the diaphysis of the femur, there was no effect on the factors again
It seems that while LIPUS might work in somehow increasing the rate of bone non-union healing, it has almost no effect on bone physeal growth. It doesn’t even work on young lab rats.
At this point, I propose that we STOP going down this line of research, on the possibility of using the Low-Intensity Pulsed Ultrasound as a possible way to increase height, especially for adults with no cartilage to work with.
Tyler-I looked over this full study
“Four-week-old Sprague–Dawley male rats (approximately 90 g) were treated for 20 min/day with ultrasound applied to the left leg during four weeks, while the right leg served as internal control”
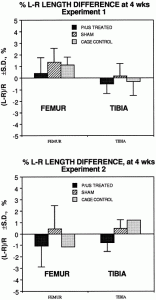 Experiment 2 is with periosteal abrasion.
Experiment 2 is with periosteal abrasion.
| Exposure time (days) |
Treatment group |
Experiment 1 (ultrasound only)
|
Experiment 2 (ultrasound + periosteal trauma)
|
| n |
Left femur (treated) |
Right femur (control) |
Left tibia (treated) |
Right tibia (control) |
n |
Left femur (treated) |
Right femur (control) |
Left tibia (treated) |
Right tibia (control) |
| 0 |
Active |
6 |
22.00 ± 0.50 |
21.82 ± 0.39 |
25.80 ± 0.87 |
25.90 ± 0.79 |
6 |
19.24 ± 0.80 |
19.11 ± 0.81 |
26.75 ± 0.70 |
26.89 ± 0.79 |
|
Sham |
6 |
21.47 ± 0.45 |
21.93 ± 0.60 |
25.65 ± 0.47 |
26.13 ± 0.90 |
6 |
19.64 ± 0.23 |
19.89 ± 0.75 |
27.25 ± 0.79 |
27.20 ± 0.93 |
|
Normal |
4 |
22.32 ± 0.25 |
21.55 ± 0.89 |
24.29 ± 0.78 |
26.07 ± 0.92 |
|
|
|
|
|
| 14 |
Active |
6 |
28.39 ± 0.71 |
28.55 ± 0.65 |
32.17 ± 0.47 |
32.35 ± 0.87 |
6 |
25.89 ± 0.70 |
26.36 ± 0.62 |
33.32 ± 0.62 |
33.49 ± 0.71 |
|
Sham |
6 |
27.91 ± 0.97 |
28.00 ± 0.60 |
31.64 ± 0.45 |
31.53 ± 0.62 |
6 |
26.77 ± 0.80 |
26.93 ± 1.09 |
34.14 ± 0.80 |
34.20 ± 0.17 |
|
Normal |
4 |
28.69 ± 0.47 |
28.52 ± 0.77 |
33.42 ± 1.53 |
32.84 ± 0.28 |
|
|
|
|
|
| 28 |
Active |
6 |
31.25 ± 0.64 |
31.07 ± 0.64 |
35.36 ± 0.41 |
35.34 ± 0.69 |
6 |
27.58 ± 1.43 |
28.72 ± 1.33 |
36.82 ± 0.59 |
36.83 ± 0.58 |
|
Sham |
6 |
31.02 ± 0.67 |
31.18 ± 0.97 |
34.49 ± 0.72 |
34.45 ± 0.81 |
6 |
28.13 ± 1.68 |
27.87 ± 1.35 |
37.20 ± 1.11 |
37.17 ± 1.01 |
|
Normal |
4 |
32.16 ± 0.73 |
32.03 ± 0.41 |
36.03 ± 0.62 |
35.89 ± 0.40 |
|
|
|
|
|
In one study I mentioned on this post on ultrasound, Pulsed Wave Ultrasound on the metatarsal was able to increase longiudinal bone growth rate.
Looking at this study:
Effects of growth hormone and ultrasound on mandibular growth in rats: MicroCT and toxicity analyses.
“Mandibular growth can be enhanced by the systemic administration of recombinant growth hormone (rGH) and/or local application of therapeutic low intensity pulsed ultrasound (LIPUS). The purpose of this study was to determine if local injection of rGH and application of LIPUS to the temporomandibular joint (TMJ) would synergistically enhance mandibular growth. In an animal study, the effect of rGH, LIPUS, and combination of rGH and LIPUS on male Sprague-Dawley rats was observed. Mandibular growth was evaluated by measuring total hemimandibular and condylar bone volume and bone surface area as well as condylar bone mineral density (BMD) after 21 days on dissected rats’ mandibles using micro-computed tomography (MicroCT). The expression of c-jun mRNA extracted from the liver of each of these rats was also quantified by real-time polymerase chain reaction to evaluate possible systemic effect of local rGH administration. Significant growth stimulation was observed in the mandibular and condylar bone of the animals treated with rGH, LIPUS, and rGH/LIPUS combined when compared with the control group. Bone volume, surface area, condylar bone mineral density, and c-jun expression were also compared between the treatment groups and the control in the liver. The results suggest that mandibular growth may be enhanced by injection of rGH or LIPUS application. The current study although showed synergetic effect of rGH and LIPUS application in increasing mandibular condylar head length, there was no significant changes in mandibular bone volume using both treatments together when compared to the two individual treatments. Moreover, combined rGH and LIPUS decreased condylar bone mineral density than each treatment separately. Future research could be directed to investigate the effects of different rGH doses and/or different LIPUS exposures parameters on lower jaw growth. ”
Both LIPUS and HGH increased c-Jun expression but HGH did it to a far greater degree.
” 8-week-old male Sprague–Dawley rats weighing 200 g”
Mandibular Condyle seems to respond to different things than other bone types(like femur and tibia) respond to stimuli though. It’s been shown that LIPUS can affect stimuli involved in height growth like the growth plate directly and mesenchymal stem cells. Maybe just specific application of LIPUS is needed. Different frequencies for example could be required to induce longitudinal bone growth.